Reactive oxygen species and oxidative stress in ocular disease: from molecular mechanisms to targeted therapies
Medical hypothesis discovery and innovation in ophthalmology,
Vol. 14 No. 3 (2025),
27 September 2025
,
Page 136-145
https://doi.org/10.51329/mehdiophthal1527
Abstract
Background: Reactive oxygen species and oxidative stress are increasingly recognized as central drivers in the development of major ocular diseases, including cataracts, age-related macular degeneration, glaucoma, and diabetic retinopathy. The eye’s unique environment—continuous light exposure, high oxygen tension, and abundant photosensitizers—renders it particularly vulnerable to ROS-mediated damage. This narrative review aims to synthesize current evidence on the molecular mechanisms of oxidative stress in ocular disease and highlight emerging therapeutic approaches.Methods: Targeted searches of PubMed, Scopus, and Google Scholar for literature published between 2000 and June 2025 were conducted. Keywords included “oxidative stress”, “reactive oxygen species”, “ocular disease”, “cataract”, “age-related macular degeneration”, “glaucoma”, and “diabetic retinopathy”. Only English-language, peer-reviewed articles were considered. Relevant primary studies, clinical trials, reviews, and experimental reports were selectively incorporated, with an emphasis on recent publications and high-impact contributions to the field.
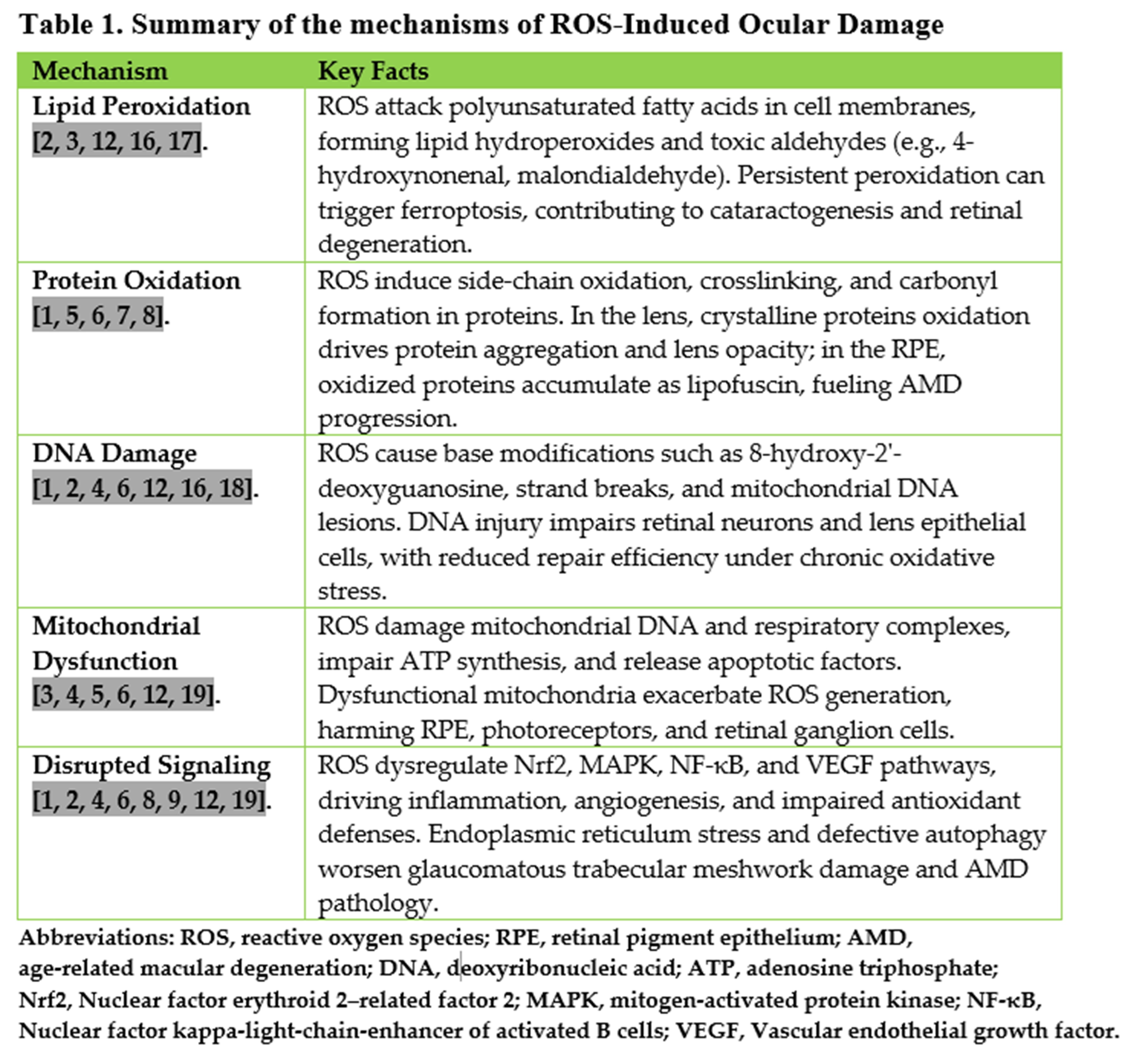
Results: Evidence consistently demonstrates that ROS induce lipid peroxidation, protein oxidation, DNA damage, mitochondrial dysfunction, and disruption of redox-sensitive cellular signaling pathways across ocular tissues. In cataracts, oxidation of crystalline proteins and glutathione depletion are primary drivers of lens opacification. In age-related macular degeneration, mitochondrial dysfunction and lipofuscin accumulation promote retinal pigment epithelium degeneration and neovascularization. Glaucoma involves both trabecular meshwork oxidative injury, contributing to elevated intraocular pressure, and mitochondrial-driven retinal ganglion cell apoptosis. In diabetic retinopathy, hyperglycemia-induced ROS overload activates pathogenic pathways, leading to microvascular damage and neuronal dysfunction. Clinical and experimental studies support antioxidant therapies as adjunctive strategies, with the strongest evidence for Age-Related Eye Disease Study-based formulations in age-related macular degeneration and promising results for agents such as Coenzyme Q10 in glaucoma and sulforaphane in diabetic retinopathy. For cataracts, supplementation trials have yielded mixed outcomes and surgery remains the definitive treatment.
Conclusions: Oxidative stress represents a unifying mechanism in the pathogenesis of vision-threatening ocular diseases. Antioxidant-based interventions show potential, particularly when integrated with existing treatment regimens, but their translation into routine practice remains limited by heterogeneous trial results and the absence of robust biomarkers for patient selection. Future research should focus on precision antioxidant therapy, leveraging stage-specific interventions, novel delivery systems, and pathway-targeted compounds, to transform ocular care from reactive management toward prevention.
- Abstract Viewed: 0 times
- Full Text PDF Downloaded: 0 times


