The Antiangiogenic Properties of Adipose-Derived Mesenchymal Stem/Stromal Cells in Corneal Neovascularization in a Rabbit Model
Medical hypothesis discovery and innovation in ophthalmology,
Vol. 9 No. 2 (2020),
4 March 2020
,
Page 74-84
Abstract
The purpose was to study the anti-angiogenic effect of adipose-derived mesenchymalstem/stromal cells (ADMSCs) on experimentally induced corneal injuries. Corneal neovascularization (NV) was induced by incising and subsequently suturing the corneal surface in 32 New Zealand rabbits. Following suturing, the rabbits were randomly allocated into 2 groups and received either phosphate-buffered saline (PBS) (control) or ADMSCs, both administered via three different routes. Digital images of the cornea were obtained two weeks’ post-incision to measure the area of neovascularized cornea. Tumor necrosis factor (TNF) was immunohistochemically assessed in the both groups. The corneal tissue was evaluated for vascular endothelial growth factor (VEGF). The extent of corneal NV in all eyes was assessed photographically by an independent observer. Fourteen days after the incisions, the degree of corneal NV was substantially decreased in the ADMSC-treated group (1.87 ± 0.9 millimeters squared, 1.4 % ± 0.67 % of corneal surface) compared to the control and PBS-treated group (4.66 ± 1.74 millimeters squared, 3.51 % ± 1.31 %, p < 0.001). ADMSCs significantly decreased injury-induced corneal NV in New Zealand rabbits two weeks’ post-treatment. This strategy has the potential for use in the control of corneal NV in vivo.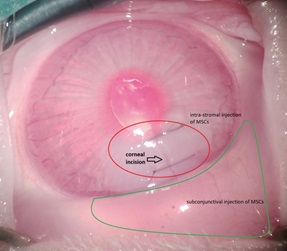
References
Meek KM, Knupp C. Corneal structure and transparency. Prog Retin Eye Res. 2015;49:1-16. doi: 10.1016/j.preteyeres.2015.07.001 pmid: 26145225
Benayoun Y, Casse G, Forte R, Dallaudiere B, Adenis JP, Robert PY. [Corneal neovascularization: epidemiological, physiopathological, and clinical features]. J Fr Ophtalmol. 2013;36(7):627-39. doi: 10.1016/j.jfo.2013.03.002 pmid: 23891320
Perez VL, Saeed AM, Tan Y, Urbieta M, Cruz-Guilloty F. The eye: A window to the soul of the immune system. J Autoimmun. 2013;45:7-14. doi: 10.1016/j.jaut.2013.06.011 pmid: 23871641
Ellenberg D, Azar DT, Hallak JA, Tobaigy F, Han KY, Jain S, et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29(3):208-48. doi: 10.1016/j.preteyeres.2010.01.002 pmid: 20100589
Cursiefen C. Immune privilege and angiogenic privilege of the cornea. Chem Immunol Allergy. 2007;92:50-7. doi: 10.1159/000099253 pmid: 17264482
Kao WW, Zhu G, Benza R, Kao CW, Ishizaki M, Wander AH. Appearance of immune cells and expression of MHC II DQ molecule by fibroblasts in alkali-burned corneas. Cornea. 1996;15(4):397-408. doi: 10.1097/00003226-199607000-00010 pmid: 8776566
Cursiefen C, Colin J, Dana R, Diaz-Llopis M, Faraj LA, Garcia-Delpech S, et al. Consensus statement on indications for anti-angiogenic therapy in the management of corneal diseases associated with neovascularisation: outcome of an expert roundtable. Br J Ophthalmol. 2012;96(1):3-9. doi: 10.1136/bjo.2011.204701 pmid: 21712359
Li Z, Burns AR, Han L, Rumbaut RE, Smith CW. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Am J Pathol. 2011;178(3):1106-16. doi: 10.1016/j.ajpath.2010.12.001 pmid: 21356362
Strömblad S, Cheresh DA. Integrins, angiogenesis and vascular cell survival. Chemistry & Biology. 1996;3(11):881-5. doi: 10.1016/s1074-5521(96)90176-3
Usui-Kusumoto K, Iwanishi H, Ichikawa K, Okada Y, Sumioka T, Miyajima M, et al. Suppression of neovascularization in corneal stroma in a TRPA1-null mouse. Exp Eye Res. 2019;181:90-7. doi: 10.1016/j.exer.2019.01.002 pmid: 30633924
Chen WS, Cao Z, Leffler H, Nilsson UJ, Panjwani N. Galectin-3 Inhibition by a Small-Molecule Inhibitor Reduces Both Pathological Corneal Neovascularization and Fibrosis. Invest Ophthalmol Vis Sci. 2017;58(1):9-20. doi: 10.1167/iovs.16-20009 pmid: 28055102
Du HT, Du LL, Tang XL, Ge HY, Liu P. Blockade of MMP-2 and MMP-9 inhibits corneal lymphangiogenesis. Graefes Arch Clin Exp Ophthalmol. 2017;255(8):1573-9. doi: 10.1007/s00417-017-3651-8 pmid: 28669039
Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16(6):593-609. doi: 10.1016/j.cytogfr.2005.04.007 pmid: 16046180
Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000;41(9):2514-22. pmid: 10937562
Kasiri A, Ghomi MR, Feghhi M, Farrahi F, Mirdehghan MS, Hedayati H. Topical Timolol Inhibits Corneal Neovascularization in Rabbits. Med Hypothesis Discov Innov Ophthalmol. 2017;6(2):39-43. pmid: 29367933
Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83(3):233-40. doi: 10.1161/01.res.83.3.233 pmid: 9710115
David Dong ZM, Aplin AC, Nicosia RF. Regulation of angiogenesis by macrophages, dendritic cells, and circulating myelomonocytic cells. Curr Pharm Des. 2009;15(4):365-79. doi: 10.2174/138161209787315783 pmid: 19199964
Woods J, Jones L, Woods C, Schneider S, Fonn D. Use of a photographic manipulation tool to assess corneal vascular response. Optom Vis Sci. 2012;89(2):215-20. doi: 10.1097/OPX.0b013e31823edec2 pmid: 22237419
Li F, Zhao SZ. Mesenchymal stem cells: Potential role in corneal wound repair and transplantation. World J Stem Cells. 2014;6(3):296-304. doi: 10.4252/wjsc.v6.i3.296 pmid: 25126379
Zhou B, Tsaknakis G, Coldwell KE, Khoo CP, Roubelakis MG, Chang CH, et al. A novel function for the haemopoietic supportive murine bone marrow MS-5 mesenchymal stromal cell line in promoting human vasculogenesis and angiogenesis. Br J Haematol. 2012;157(3):299-311. doi: 10.1111/j.1365-2141.2012.09050.x pmid: 22324374
Watt SM, Gullo F, van der Garde M, Markeson D, Camicia R, Khoo CP, et al. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull. 2013;108:25-53. doi: 10.1093/bmb/ldt031 pmid: 24152971
Otero-Vinas M, Falanga V. Mesenchymal Stem Cells in Chronic Wounds: The Spectrum from Basic to Advanced Therapy. Adv Wound Care (New Rochelle). 2016;5(4):149-63. doi: 10.1089/wound.2015.0627 pmid: 27076993
Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, et al. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26(4):1047-55. doi: 10.1634/stemcells.2007-0737 pmid: 18192235
Karathanasis V, Petrakis S, Topouridou K, Koliakou E, Koliakos G, Demiri E. Intradermal injection of GFP-producing adipose stromal cells promotes survival of random-pattern skin flaps in rats. European J Plastic Surgery. 2013;36(5):281-8. doi: 10.1007/s00238-013-0810-y
Jones EA, English A, Kinsey SE, Straszynski L, Emery P, Ponchel F, et al. Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin Cytom. 2006;70(6):391-9. doi: 10.1002/cyto.b.20118 pmid: 16977637
Statement AfRiVaO. research ftuoaioav. http://www.arvo.org/About_ARVO/Policies/Statement_for_the_Use_of_Animals_in_Ophthalmic_and_Visual_Research/#Animal.
Matsuda A, Tagawa Y, Matsuda H, Nishihira J. Expression of macrophage migration inhibitory factor in corneal wound healing in rats. Invest Ophthalmol Vis Sci. 1997;38(8):1555-62. pmid: 9224283
Demirayak B, Yuksel N, Celik OS, Subasi C, Duruksu G, Unal ZS, et al. Effect of bone marrow and adipose tissue-derived mesenchymal stem cells on the natural course of corneal scarring after penetrating injury. Exp Eye Res. 2016;151:227-35. doi: 10.1016/j.exer.2016.08.011 pmid: 27567556
Kim TI, Kim SW, Kim S, Kim T, Kim EK. Inhibition of experimental corneal neovascularization by using subconjunctival injection of bevacizumab (Avastin). Cornea. 2008;27(3):349-52. doi: 10.1097/ICO.0b013e31815cf67d pmid: 18362666
Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214-22. doi: 10.1016/j.scr.2009.12.003 pmid: 20138817
Peh GS, Beuerman RW, Colman A, Tan DT, Mehta JS. Human corneal endothelial cell expansion for corneal endothelium transplantation: an overview. Transplantation. 2011;91(8):811-9. doi: 10.1097/TP.0b013e3182111f01 pmid: 21358368
Feizi S. Corneal endothelial cell dysfunction: etiologies and management. Ther Adv Ophthalmol. 2018;10:2515841418815802. doi: 10.1177/2515841418815802 pmid: 30560230
Oh JY, Lee RH, Yu JM, Ko JH, Lee HJ, Ko AY, et al. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther. 2012;20(11):2143-52. doi: 10.1038/mt.2012.165 pmid: 22929658
Liu H, Zhang J, Liu CY, Wang IJ, Sieber M, Chang J, et al. Cell therapy of congenital corneal diseases with umbilical mesenchymal stem cells: lumican null mice. PLoS One. 2010;5(5):e10707. doi: 10.1371/journal.pone.0010707 pmid: 20502663
Yao L, Li ZR, Su WR, Li YP, Lin ML, Zhang WX, et al. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS One. 2012;7(2):e30842. doi: 10.1371/journal.pone.0030842 pmid: 22363499
Ye J, Yao K, Kim JC. Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: engraftment and involvement in wound healing. Eye (Lond). 2006;20(4):482-90. doi: 10.1038/sj.eye.6701913 pmid: 15895027
Zajicova A, Pokorna K, Lencova A, Krulova M, Svobodova E, Kubinova S, et al. Treatment of ocular surface injuries by limbal and mesenchymal stem cells growing on nanofiber scaffolds. Cell Transplant. 2010;19(10):1281-90. doi: 10.3727/096368910X509040 pmid: 20573307
Ghazaryan E, Zhang Y, He Y, Liu X, Li Y, Xie J, et al. Mesenchymal stem cells in corneal neovascularization: Comparison of different application routes. Mol Med Rep. 2016;14(4):3104-12. doi: 10.3892/mmr.2016.5621 pmid: 27514011
Oh JY, Roddy GW, Choi H, Lee RH, Ylostalo JH, Rosa RH, Jr., et al. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci U S A. 2010;107(39):16875-80. doi: 10.1073/pnas.1012451107 pmid: 20837529
Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143(2):181-96. doi: 10.1016/j.pharmthera.2014.02.013 pmid: 24594234
Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells. 2015;33(7):2158-68. doi: 10.1002/stem.1771 pmid: 24964196
Song HB, Park SY, Ko JH, Park JW, Yoon CH, Kim DH, et al. Mesenchymal Stromal Cells Inhibit Inflammatory Lymphangiogenesis in the Cornea by Suppressing Macrophage in a TSG-6-Dependent Manner. Mol Ther. 2018;26(1):162-72. doi: 10.1016/j.ymthe.2017.09.026 pmid: 29301108
Wang LJ, Liu LP, Gu XL, Wang M, Liu LM. Implantation of adipose-derived stem cells cures the optic nerve injury on rats through inhibiting the expression of inflammation factors in the TLR4 signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22(5):1196-202. doi: 10.26355/eurrev_201803_14458 pmid: 29565474
Mo JS, Matsukawa A, Ohkawara S, Yoshinaga M. Involvement of TNF alpha, IL-1 beta and IL-1 receptor antagonist in LPS-induced rabbit uveitis. Exp Eye Res. 1998;66(5):547-57. doi: 10.1006/exer.1997.0451 pmid: 9628802
Ferrari G, Bignami F, Rama P. Tumor necrosis factor-alpha inhibitors as a treatment of corneal hemangiogenesis and lymphangiogenesis. Eye Contact Lens. 2015;41(2):72-6. doi: 10.1097/ICL.0000000000000071 pmid: 25503908
Wagner W, Ho AD, Zenke M. Different facets of aging in human mesenchymal stem cells. Tissue Eng Part B Rev. 2010;16(4):445-53. doi: 10.1089/ten.TEB.2009.0825 pmid: 20196648
Liu W, Liu Y, Liu H, Luo Y, Xu J. [Differentiation of adipose-derived mesenchymal stem cells after transfection with Pax6 gene]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2014;28(8):1004-8. pmid: 25417317
Sun J, Liu WH, Deng FM, Luo YH, Wen K, Zhang H, et al. Differentiation of rat adipose-derived mesenchymal stem cells into corneal-like epithelial cells driven by PAX6. Exp Ther Med. 2018;15(2):1424-32. doi: 10.3892/etm.2017.5576 pmid: 29434727
Cejkova J, Trosan P, Cejka C, Lencova A, Zajicova A, Javorkova E, et al. Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface. Exp Eye Res. 2013;116:312-23. doi: 10.1016/j.exer.2013.10.002 pmid: 24145108
Al-Jaibaji O, Swioklo S, Connon CJ. Mesenchymal stromal cells for ocular surface repair. Expert Opin Biol Ther. 2019;19(7):643-53. doi: 10.1080/14712598.2019.1607836 pmid: 30979344
Agorogiannis GI, Alexaki VI, Castana O, Kymionis GD. Topical application of autologous adipose-derived mesenchymal stem cells (MSCs) for persistent sterile corneal epithelial defect. Graefes Arch Clin Exp Ophthalmol. 2012;250(3):455-7. doi: 10.1007/s00417-011-1841-3 pmid: 22012407
- Abstract Viewed: 569 times
- Full Text PDF Downloaded: 387 times


