A Review of Last Decade Developments on Epiretinal Membrane Pathogenesis
Medical hypothesis discovery and innovation in ophthalmology,
Vol. 9 No. 2 (2020),
4 March 2020
,
Page 91-110
Abstract
Epiretinal membrane (ERM) is a pathologic tissue that develops at the vitreoretinal interface. ERM is responsible for pathological changes of vision with varying degrees of clinical significance. It is either idiopathic or secondary to a wide variety of diseases such as proliferative diabetic retinopathy (PDR) and proliferative vitreoretinopathy (PVR). A great variation in the prevalence of idiopathic ERM among different ethnic groups proposed that genetic and lifestyle factors may play a role in ERM occurrence. Histopathological studies demonstrate that various cell types including retinal pigment epithelium (RPE) cells, fibrocytes, fibrous astrocytes, myofibroblast-like cells, glial cells, endothelial cells (ECs) and macrophages, as well as trophic and transcription factors, including transforming growth factor (TGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) etc., are directly or indirectly involved in the pathogenesis of idiopathic or secondary ERMs. These processes are driven (on the last count) by more than 50 genes, such as Tumor Necrosis Factor (TNF), CCL2 ((chemokine (C-C motif) ligand 2)), MALAT1, transforming growth factor (TGF)-beta1, TGF-beta2, Interleukin-6 (IL-6), IL-10, VEGF and glial fibrillary acidic protein (GFAP), some of which have been studied more intensely than others. The present paper tried to summarize, highlight and cross-correlate the major findings made in the last decade on the function of these genes and their association with different types of cells, genes and gene expression products in the ERM formation.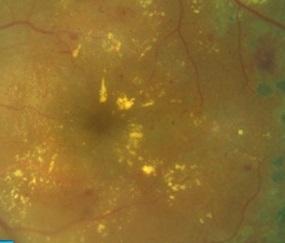
References
Smiddy WE, Maguire AM, Green WR, Michels RG, de la Cruz Z, Enger C, et al. Idiopathic epiretinal membranes: ultrastructural characteristics and clinicopathologic correlation. 1989. Retina. 2005;25(5 Suppl):811-20; discussion 21. doi: 10.1097/00006982-200507001-00012 pmid: 16049366
Yazici AT, Alagoz N, Celik HU, Bozkurt E, Alagoz C, Cakir M, et al. Idiopathic and secondary epiretinal membranes: do they differ in terms of morphology? An optical coherence tomography-based study. Retina. 2011;31(4):779-84. doi: 10.1097/IAE.0b013e3181ef8786 pmid: 21836405
McCarty DJ, Mukesh BN, Chikani V, Wang JJ, Mitchell P, Taylor HR, et al. Prevalence and associations of epiretinal membranes in the visual impairment project. Am J Ophthalmol. 2005;140(2):288-94. doi: 10.1016/j.ajo.2005.03.032 pmid: 16023066
Russo A, Ragusa M, Barbagallo C, Longo A, Avitabile T, Uva MG, et al. miRNAs in the vitreous humor of patients affected by idiopathic epiretinal membrane and macular hole. PLoS One. 2017;12(3):e0174297. doi: 10.1371/journal.pone.0174297 pmid: 28328945
Charles S. Techniques and tools for dissection of epiretinal membranes. Graefes Arch Clin Exp Ophthalmol. 2003;241(5):347-52. doi: 10.1007/s00417-003-0624-x pmid: 12682840
George B, Chen S, Chaudhary V, Gonder J, Chakrabarti S. Extracellular matrix proteins in epiretinal membranes and in diabetic retinopathy. Curr Eye Res. 2009;34(2):134-44. doi: 10.1080/02713680802585946 pmid: 19219685
Fraser-Bell S, Guzowski M, Rochtchina E, Wang JJ, Mitchell P. Five-year cumulative incidence and progression of epiretinal membranes: the Blue Mountains Eye Study. Ophthalmology. 2003;110(1):34-40. doi: 10.1016/s0161-6420(02)01443-4 pmid: 12511343
Wilkins JR, Puliafito CA, Hee MR, Duker JS, Reichel E, Coker JG, et al. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology. 1996;103(12):2142-51. doi: 10.1016/s0161-6420(96)30377-1 pmid: 9003350
Iannetti L, Accorinti M, Malagola R, Bozzoni-Pantaleoni F, Da Dalt S, Nicoletti F, et al. Role of the intravitreal growth factors in the pathogenesis of idiopathic epiretinal membrane. Invest Ophthalmol Vis Sci. 2011;52(8):5786-9. doi: 10.1167/iovs.10-7116 pmid: 21693611
Kampik A, Kenyon KR, Michels RG, Green WR, de la Cruz ZC. Epiretinal and vitreous membranes. Comparative study of 56 cases. Arch Ophthalmol. 1981;99(8):1445-54. doi: 10.1001/archopht.1981.03930020319025 pmid: 7020665
Duan XR, Liang YB, Friedman DS, Sun LP, Wei WB, Wang JJ, et al. Prevalence and associations of epiretinal membranes in a rural Chinese adult population: the Handan Eye Study. Invest Ophthalmol Vis Sci. 2009;50(5):2018-23. doi: 10.1167/iovs.08-2624 pmid: 19074799
Oberstein SY, Byun J, Herrera D, Chapin EA, Fisher SK, Lewis GP. Cell proliferation in human epiretinal membranes: characterization of cell types and correlation with disease condition and duration. Mol Vis. 2011;17:1794-805. pmid: 21750605
Koh V, Cheung CY, Wong WL, Cheung CM, Wang JJ, Mitchell P, et al. Prevalence and risk factors of epiretinal membrane in Asian Indians. Invest Ophthalmol Vis Sci. 2012;53(2):1018-22. doi: 10.1167/iovs.11-8557 pmid: 22247478
Stevenson W, Prospero Ponce CM, Agarwal DR, Gelman R, Christoforidis JB. Epiretinal membrane: optical coherence tomography-based diagnosis and classification. Clin Ophthalmol. 2016;10:527-34. doi: 10.2147/OPTH.S97722 pmid: 27099458
Joshi M, Agrawal S, Christoforidis JB. Inflammatory mechanisms of idiopathic epiretinal membrane formation. Mediators Inflamm. 2013;2013:192582. doi: 10.1155/2013/192582 pmid: 24324293
Pollreisz A, Funk M, Breitwieser FP, Parapatics K, Sacu S, Georgopoulos M, et al. Quantitative proteomics of aqueous and vitreous fluid from patients with idiopathic epiretinal membranes. Exp Eye Res. 2013;108:48-58. doi: 10.1016/j.exer.2012.11.010 pmid: 23201028
Asato R, Yoshida S, Ogura A, Nakama T, Ishikawa K, Nakao S, et al. Comparison of gene expression profile of epiretinal membranes obtained from eyes with proliferative vitreoretinopathy to that of secondary epiretinal membranes. PLoS One. 2013;8(1):e54191. doi: 10.1371/journal.pone.0054191 pmid: 23372684
Bu SC, Kuijer R, van der Worp RJ, Postma G, Renardel de Lavalette VW, Li XR, et al. Immunohistochemical Evaluation of Idiopathic Epiretinal Membranes and In Vitro Studies on the Effect of TGF-beta on Muller Cells. Invest Ophthalmol Vis Sci. 2015;56(11):6506-14. doi: 10.1167/iovs.14-15971 pmid: 26447986
Bu SC, Kuijer R, Li XR, Hooymans JM, Los LI. Idiopathic epiretinal membrane. Retina. 2014;34(12):2317-35. doi: 10.1097/IAE.0000000000000349 pmid: 25360790
Romaniuk D, Kimsa MW, Strzalka-Mrozik B, Kimsa MC, Kabiesz A, Romaniuk W, et al. Gene expression of IGF1, IGF1R, and IGFBP3 in epiretinal membranes of patients with proliferative diabetic retinopathy: preliminary study. Mediators Inflamm. 2013;2013:986217. doi: 10.1155/2013/986217 pmid: 24379526
Yamaji Y, Yoshida S, Ishikawa K, Sengoku A, Sato K, Yoshida A, et al. TEM7 (PLXDC1) in neovascular endothelial cells of fibrovascular membranes from patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49(7):3151-7. doi: 10.1167/iovs.07-1249 pmid: 18316703
Chang W, Lajko M, Fawzi AA. Endothelin-1 is associated with fibrosis in proliferative diabetic retinopathy membranes. PLoS One. 2018;13(1):e0191285. doi: 10.1371/journal.pone.0191285 pmid: 29351334
Bringmann A, Wiedemann P. Involvement of Muller glial cells in epiretinal membrane formation. Graefes Arch Clin Exp Ophthalmol. 2009;247(7):865-83. doi: 10.1007/s00417-009-1082-x pmid: 19415318
Stafiej J, Kazmierczak K, Linkowska K, Zuchowski P, Grzybowski T, Malukiewicz G. Evaluation of TGF-Beta 2 and VEGFalpha Gene Expression Levels in Epiretinal Membranes and Internal Limiting Membranes in the Course of Retinal Detachments, Proliferative Diabetic Retinopathy, Macular Holes, and Idiopathic Epiretinal Membranes. J Ophthalmol. 2018;2018:8293452. doi: 10.1155/2018/8293452 pmid: 29850215
Roth AM, Foos RY. Surface wrinkling retinopathy in eyes enucleated at autopsy. Trans Am Acad Ophthalmol Otolaryngol. 1971;75(5):1047-58. pmid: 5097819
Nam KY, Kim JY. Effect of internal limiting membrane peeling on the development of epiretinal membrane after pars plana vitrectomy for primary rhegmatogenous retinal detachment. Retina. 2015;35(5):880-5. doi: 10.1097/IAE.0000000000000421 pmid: 25545479
Cheung N, Tan SP, Lee SY, Cheung GCM, Tan G, Kumar N, et al. Prevalence and risk factors for epiretinal membrane: the Singapore Epidemiology of Eye Disease study. Br J Ophthalmol. 2017;101(3):371-6. doi: 10.1136/bjophthalmol-2016-308563 pmid: 27343209
Aung KZ, Makeyeva G, Adams MK, Chong EW, Busija L, Giles GG, et al. The prevalence and risk factors of epiretinal membranes: the Melbourne Collaborative Cohort Study. Retina. 2013;33(5):1026-34. doi: 10.1097/IAE.0b013e3182733f25 pmid: 23400080
Yu J, Feng L, Wu Y, Wang H, Ba J, Zhu W, et al. Vitreous proteomic analysis of idiopathic epiretinal membranes. Mol Biosyst. 2014;10(10):2558-66. doi: 10.1039/c4mb00240g pmid: 25014768
Foos RY. Vitreoretinal juncture--simple epiretinal membranes. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1974;189(4):231-50. doi: 10.1007/bf02384852 pmid: 4365862
Fraser-Bell S, Ying-Lai M, Klein R, Varma R, Los Angeles Latino Eye S. Prevalence and associations of epiretinal membranes in latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2004;45(6):1732-6. doi: 10.1167/iovs.03-1295 pmid: 15161833
Arichika S, Hangai M, Yoshimura N. Correlation between thickening of the inner and outer retina and visual acuity in patients with epiretinal membrane. Retina. 2010;30(3):503-8. doi: 10.1097/IAE.0b013e3181bd2d65 pmid: 19952992
Mitchell P, Smith W, Chey T, Wang JJ, Chang A. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104(6):1033-40. doi: 10.1016/s0161-6420(97)30190-0 pmid: 9186446
Tadayoni R, Paques M, Massin P, Mouki-Benani S, Mikol J, Gaudric A. Dissociated optic nerve fiber layer appearance of the fundus after idiopathic epiretinal membrane removal. Ophthalmology. 2001;108(12):2279-83. doi: 10.1016/s0161-6420(01)00856-9 pmid: 11733271
Lu Q, Ma Y, Xu YS, Jiang YR. Apelin in epiretinal membranes of patients with proliferative diabetic retinopathy. Mol Vis. 2014;20:1122-31. pmid: 25324682
Kwok A, Lai TY, Yuen KS. Epiretinal membrane surgery with or without internal limiting membrane peeling. Clin Exp Ophthalmol. 2005;33(4):379-85. doi: 10.1111/j.1442-9071.2005.01015.x pmid: 16033350
Jeon S, Baek J, Lee WK. Gli1 Expression in Human Epiretinal Membranes. Invest Ophthalmol Vis Sci. 2017;58(1):651-9. doi: 10.1167/iovs.16-20409 pmid: 28134963
Kawasaki R, Wang JJ, Mitchell P, Aung T, Saw SM, Wong TY, et al. Racial difference in the prevalence of epiretinal membrane between Caucasians and Asians. Br J Ophthalmol. 2008;92(10):1320-4. doi: 10.1136/bjo.2008.144626 pmid: 18658173
Semeraro F, Morescalchi F, Duse S, Gambicorti E, Russo A, Costagliola C. Current Trends about Inner Limiting Membrane Peeling in Surgery for Epiretinal Membranes. J Ophthalmol. 2015;2015:671905. doi: 10.1155/2015/671905 pmid: 26425352
Ng CH, Cheung N, Wang JJ, Islam AF, Kawasaki R, Meuer SM, et al. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology. 2011;118(4):694-9. doi: 10.1016/j.ophtha.2010.08.009 pmid: 21035863
Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. 1994;92:403-25; discussion 25-30. pmid: 7886875
Appiah AP, Hirose T. Secondary causes of premacular fibrosis. Ophthalmology. 1989;96(3):389-92. doi: 10.1016/s0161-6420(89)32881-8 pmid: 2710531
Yoshida S, Ogura A, Ishikawa K, Yoshida A, Kohno R, Yamaji Y, et al. Gene expression profile of fibrovascular membranes from patients with proliferative diabetic retinopathy. Br J Ophthalmol. 2010;94(6):795-801. doi: 10.1136/bjo.2009.167072 pmid: 19919945
Bu SC, Kuijer R, van der Worp RJ, Huiskamp EA, Renardel de Lavalette VW, Li XR, et al. Glial cells and collagens in epiretinal membranes associated with idiopathic macular holes. Retina. 2014;34(5):897-906. doi: 10.1097/IAE.0000000000000013 pmid: 24077090
Simo R, Carrasco E, Garcia-Ramirez M, Hernandez C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev. 2006;2(1):71-98. doi: 10.2174/157339906775473671 pmid: 18220619
Wiedemann P. Growth factors in retinal diseases: proliferative vitreoretinopathy, proliferative diabetic retinopathy, and retinal degeneration. Surv Ophthalmol. 1992;36(5):373-84. doi: 10.1016/0039-6257(92)90115-a pmid: 1566240
Myojin S, Yoshimura T, Yoshida S, Takeda A, Murakami Y, Kawano Y, et al. Gene Expression Analysis of the Irrigation Solution Samples Collected during Vitrectomy for Idiopathic Epiretinal Membrane. PLoS One. 2016;11(10):e0164355. doi: 10.1371/journal.pone.0164355 pmid: 27736918
Foos RY. Vitreoretinal juncture; epiretinal membranes and vitreous. Invest Ophthalmol Vis Sci. 1977;16(5):416-22. pmid: 852943
Minchiotti S, Stampachiacchiere B, Micera A, Lambiase A, Ripandelli G, Billi B, et al. Human idiopathic epiretinal membranes express NGF and NGF receptors. Retina. 2008;28(4):628-37. doi: 10.1097/IAE.0b013e31815ec275 pmid: 18398367
Xu J, Zhong H, Cui L, Lan Q, Chen L, He W, et al. Expression of wild-type p53-induced phosphatase 1 in diabetic epiretinal membranes. Oncotarget. 2017;8(22):35532-41. doi: 10.18632/oncotarget.16683 pmid: 28402943
Mitamura Y, Harada T, Harada C, Ohtsuka K, Kotake S, Ohno S, et al. NF-kappaB in epiretinal membranes after human diabetic retinopathy. Diabetologia. 2003;46(5):699-703. doi: 10.1007/s00125-003-1084-x pmid: 12743697
Shao Y, Dong LJ, Takahashi Y, Chen J, Liu X, Chen Q, et al. miRNA-451a regulates RPE function through promoting mitochondrial function in proliferative diabetic retinopathy. Am J Physiol Endocrinol Metab. 2019;316(3):E443-E52. doi: 10.1152/ajpendo.00360.2018 pmid: 30576241
He S, Kumar SR, Zhou P, Krasnoperov V, Ryan SJ, Gill PS, et al. Soluble EphB4 inhibition of PDGF-induced RPE migration in vitro. Invest Ophthalmol Vis Sci. 2010;51(1):543-52. doi: 10.1167/iovs.09-3475 pmid: 19696168
Kociok N, Joussen AM. Enhanced expression of the complement factor H mRNA in proliferating human RPE cells. Graefes Arch Clin Exp Ophthalmol. 2010;248(8):1145-53. doi: 10.1007/s00417-010-1371-4 pmid: 20376478
Yu J, Liu F, Cui SJ, Liu Y, Song ZY, Cao H, et al. Vitreous proteomic analysis of proliferative vitreoretinopathy. Proteomics. 2008;8(17):3667-78. doi: 10.1002/pmic.200700824 pmid: 18752205
Bellhorn MB, Friedman AH, Wise GN, Henkind P. Ultrastructure and clinicopathologic correlation of idiopathic preretinal macular fibrosis. Am J Ophthalmol. 1975;79(3):366-73. doi: 10.1016/0002-9394(75)90608-x pmid: 1121993
Luo W, Hu L, Li W, Xu G, Xu L, Zhang C, et al. Epo inhibits the fibrosis and migration of Muller glial cells induced by TGF-beta and high glucose. Graefes Arch Clin Exp Ophthalmol. 2016;254(5):881-90. doi: 10.1007/s00417-016-3290-5 pmid: 26907931
Walshe R, Esser P, Wiedemann P, Heimann K. Proliferative retinal diseases: myofibroblasts cause chronic vitreoretinal traction. Br J Ophthalmol. 1992;76(9):550-2. doi: 10.1136/bjo.76.9.550 pmid: 1420061
Guidry C. The role of Muller cells in fibrocontractive retinal disorders. Prog Retin Eye Res. 2005;24(1):75-86. doi: 10.1016/j.preteyeres.2004.07.001 pmid: 15555527
Schumann RG, Eibl KH, Zhao F, Scheerbaum M, Scheler R, Schaumberger MM, et al. Immunocytochemical and ultrastructural evidence of glial cells and hyalocytes in internal limiting membrane specimens of idiopathic macular holes. Invest Ophthalmol Vis Sci. 2011;52(11):7822-34. doi: 10.1167/iovs.11-7514 pmid: 21900375
Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397-424. doi: 10.1016/j.preteyeres.2006.05.003 pmid: 16839797
Harada C, Harada T, Mitamura Y, Quah HM, Ohtsuka K, Kotake S, et al. Diverse NF-kappaB expression in epiretinal membranes after human diabetic retinopathy and proliferative vitreoretinopathy. Mol Vis. 2004;10:31-6. pmid: 14737065
Zandi S, Tappeiner C, Pfister IB, Despont A, Rieben R, Garweg JG. Vitreal Cytokine Profile Differences Between Eyes With Epiretinal Membranes or Macular Holes. Invest Ophthalmol Vis Sci. 2016;57(14):6320-6. doi: 10.1167/iovs.16-20657 pmid: 27893098
Zhang Y, Wang JH, Zhang YY, Wang YZ, Wang J, Zhao Y, et al. Deletion of interleukin-6 alleviated interstitial fibrosis in streptozotocin-induced diabetic cardiomyopathy of mice through affecting TGFbeta1 and miR-29 pathways. Sci Rep. 2016;6:23010. doi: 10.1038/srep23010 pmid: 26972749
Kluge A, Zimmermann R, Weihrauch D, Mohri M, Sack S, Schaper J, et al. Coordinate expression of the insulin-like growth factor system after microembolisation in porcine heart. Cardiovasc Res. 1997;33(2):324-31. doi: 10.1016/s0008-6363(96)00236-2 pmid: 9074696
Harada C, Mitamura Y, Harada T. The role of cytokines and trophic factors in epiretinal membranes: involvement of signal transduction in glial cells. Prog Retin Eye Res. 2006;25(2):149-64. doi: 10.1016/j.preteyeres.2005.09.001 pmid: 16377232
Kohno RI, Hata Y, Kawahara S, Kita T, Arita R, Mochizuki Y, et al. Possible contribution of hyalocytes to idiopathic epiretinal membrane formation and its contraction. Br J Ophthalmol. 2009;93(8):1020-6. doi: 10.1136/bjo.2008.155069 pmid: 19429593
Ikeda T, Homma Y, Nisida K, Hirase K, Sotozono C, Kinoshita S, et al. Expression of transforming growth factor-beta s and their receptors by human retinal glial cells. Curr Eye Res. 1998;17(5):546-50. doi: 10.1076/ceyr.17.5.546.5197 pmid: 9617551
Lazarus HS, Schoenfeld CL, Fekrat S, Cohen S, Carol A, Hageman GS, et al. Hyalocytes synthesize and secrete inhibitors of retinal pigment epithelial cell proliferation in vitro. Arch Ophthalmol. 1996;114(6):731-6. doi: 10.1001/archopht.1996.01100130723015 pmid: 8639087
Abukawa H, Tomi M, Kiyokawa J, Hori S, Kondo T, Terasaki T, et al. Modulation of retinal capillary endothelial cells by Muller glial cell-derived factors. Mol Vis. 2009;15:451-7. pmid: 19247458
Lupien CB, Bolduc C, Landreville S, Salesse C. Comparison between the gene expression profile of human Muller cells and two spontaneous Muller cell lines. Invest Ophthalmol Vis Sci. 2007;48(11):5229-42. doi: 10.1167/iovs.07-0122 pmid: 17962478
Vinores SA, Campochiaro PA, Conway BP. Ultrastructural and electron-immunocytochemical characterization of cells in epiretinal membranes. Invest Ophthalmol Vis Sci. 1990;31(1):14-28. pmid: 1688833
Tano K, Mizuno R, Okada T, Rakwal R, Shibato J, Masuo Y, et al. MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. FEBS Lett. 2010;584(22):4575-80. doi: 10.1016/j.febslet.2010.10.008 pmid: 20937273
Wang XC, Jobin C, Allen JB, Roberts WL, Jaffe GJ. Suppression of NF-kappaB-dependent proinflammatory gene expression in human RPE cells by a proteasome inhibitor. Invest Ophthalmol Vis Sci. 1999;40(2):477-86. pmid: 9950608
Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141-79. doi: 10.1146/annurev.iy.12.040194.001041 pmid: 8011280
Koyama Y. Signaling molecules regulating phenotypic conversions of astrocytes and glial scar formation in damaged nerve tissues. Neurochem Int. 2014;78:35-42. doi: 10.1016/j.neuint.2014.08.005 pmid: 25180676
Fiscella M, Zhang H, Fan S, Sakaguchi K, Shen S, Mercer WE, et al. Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci U S A. 1997;94(12):6048-53. doi: 10.1073/pnas.94.12.6048 pmid: 9177166
Goloudina AR, Kochetkova EY, Pospelova TV, Demidov ON. Wip1 phosphatase: between p53 and MAPK kinases pathways. Oncotarget. 2016;7(21):31563-71. doi: 10.18632/oncotarget.7325 pmid: 26883196
Yoshida-Hata N, Mitamura Y, Oshitari T, Namekata K, Harada C, Harada T, et al. Transcription factor, SP1, in epiretinal membranes of patients with proliferative diabetic retinopathy. Diabetes Res Clin Pract. 2010;87(3):e26-8. doi: 10.1016/j.diabres.2009.12.008 pmid: 20047772
Mitamura Y, Harada C, Harada T. Role of cytokines and trophic factors in the pathogenesis of diabetic retinopathy. Curr Diabetes Rev. 2005;1(1):73-81. doi: 10.2174/1573399052952596 pmid: 18220584
Canfield AE, Schor AM. Evidence that tenascin and thrombospondin-1 modulate sprouting of endothelial cells. J Cell Sci. 1995;108 ( Pt 2):797-809. pmid: 7539439
Okada M, Ogino N, Matsumura M, Honda Y, Nagai Y. Histological and immunohistochemical study of idiopathic epiretinal membrane. Ophthalmic Res. 1995;27(2):118-28. doi: 10.1159/000267612 pmid: 8538984
Kritzenberger M, Junglas B, Framme C, Helbig H, Gabel VP, Fuchshofer R, et al. Different collagen types define two types of idiopathic epiretinal membranes. Histopathology. 2011;58(6):953-65. doi: 10.1111/j.1365-2559.2011.03820.x pmid: 21480957
Sabatelli P, Bonaldo P, Lattanzi G, Braghetta P, Bergamin N, Capanni C, et al. Collagen VI deficiency affects the organization of fibronectin in the extracellular matrix of cultured fibroblasts. Matrix Biol. 2001;20(7):475-86. doi: 10.1016/s0945-053x(01)00160-3 pmid: 11691587
Groulx JF, Gagne D, Benoit YD, Martel D, Basora N, Beaulieu JF. Collagen VI is a basement membrane component that regulates epithelial cell-fibronectin interactions. Matrix Biol. 2011;30(3):195-206. doi: 10.1016/j.matbio.2011.03.002 pmid: 21406227
Elner SG, Elner VM, Jaffe GJ, Stuart A, Kunkel SL, Strieter RM. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995;14(11):1045-53. doi: 10.3109/02713689508998529 pmid: 8585935
El-Ghrably IA, Dua HS, Orr GM, Fischer D, Tighe PJ. Detection of cytokine mRNA production in infiltrating cells in proliferative vitreoretinopathy using reverse transcription polymerase chain reaction. Br J Ophthalmol. 1999;83(11):1296-9. doi: 10.1136/bjo.83.11.1296 pmid: 10535861
Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277(1):C1-9. doi: 10.1152/ajpcell.1999.277.1.C1 pmid: 10409103
Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83(6):1323-33. doi: 10.1189/jlb.1107782 pmid: 18332234
Eastlake K, Banerjee PJ, Angbohang A, Charteris DG, Khaw PT, Limb GA. Muller glia as an important source of cytokines and inflammatory factors present in the gliotic retina during proliferative vitreoretinopathy. Glia. 2016;64(4):495-506. doi: 10.1002/glia.22942 pmid: 26556395
Liu P, Zhang C, Feng JB, Zhao YX, Wang XP, Yang JM, et al. Cross talk among Smad, MAPK, and integrin signaling pathways enhances adventitial fibroblast functions activated by transforming growth factor-beta1 and inhibited by Gax. Arterioscler Thromb Vasc Biol. 2008;28(4):725-31. doi: 10.1161/ATVBAHA.107.159889 pmid: 18187669
Yamamoto T, Akabane N, Takeuchi S. Vitrectomy for diabetic macular edema: the role of posterior vitreous detachment and epimacular membrane. Am J Ophthalmol. 2001;132(3):369-77. doi: 10.1016/s0002-9394(01)01050-9 pmid: 11530050
Kuiper EJ, de Smet MD, van Meurs JC, Tan HS, Tanck MW, Oliver N, et al. Association of connective tissue growth factor with fibrosis in vitreoretinal disorders in the human eye. Arch Ophthalmol. 2006;124(10):1457-62. doi: 10.1001/archopht.124.10.1457 pmid: 17030714
Hashimoto R, Jiang M, Shiba T, Hiruta N, Takahashi M, Higashi M, et al. Soluble form of LR11 is highly increased in the vitreous fluids of patients with idiopathic epiretinal membrane. Graefes Arch Clin Exp Ophthalmol. 2017;255(5):885-91. doi: 10.1007/s00417-017-3585-1 pmid: 28102455
Lesnik Oberstein SY, Lewis GP, Dutra T, Fisher SK. Evidence that neurites in human epiretinal membranes express melanopsin, calretinin, rod opsin and neurofilament protein. Br J Ophthalmol. 2011;95(2):266-72. doi: 10.1136/bjo.2010.180679 pmid: 20971788
Rollin R, Mediero A, Martinez-Montero JC, Roldan-Pallares M, Suarez-Leoz M, Vidal-Fernandez P, et al. Atrial natriuretic peptide in the vitreous humor and epiretinal membranes of patients with proliferative diabetic retinopathy. Mol Vis. 2004;10:450-7. pmid: 15273657
Dong Z, Kase S, Ando R, Fukuhara J, Saito W, Kanda A, et al. Alphab-crystallin expression in epiretinal membrane of human proliferative diabetic retinopathy. Retina. 2012;32(6):1190-6. doi: 10.1097/IAE.0b013e318233ab9c pmid: 22371118
Lee MJ, Shin DH, Ko KI, Koo HM, Kim CH, Doh FM, et al. Association between the ratio of insulin-like growth factor-I to insulin-like growth factor binding protein-3 and inflammation in incident automated peritoneal dialysis patients. Growth Horm IGF Res. 2013;23(5):170-4. doi: 10.1016/j.ghir.2013.06.004 pmid: 23850448
Vereb Z, Lumi X, Andjelic S, Globocnik-Petrovic M, Urbancic M, Hawlina M, et al. Functional and molecular characterization of ex vivo cultured epiretinal membrane cells from human proliferative diabetic retinopathy. Biomed Res Int. 2013;2013:492376. doi: 10.1155/2013/492376 pmid: 24195074
Vinores SA, Henderer JD, Mahlow J, Chiu C, Derevjanik NL, Larochelle W, et al. Isoforms of platelet-derived growth factor and its receptors in epiretinal membranes: immunolocalization to retinal pigmented epithelial cells. Exp Eye Res. 1995;60(6):607-19. doi: 10.1016/s0014-4835(05)80003-x pmid: 7641844
Cui J, Lei H, Samad A, Basavanthappa S, Maberley D, Matsubara J, et al. PDGF receptors are activated in human epiretinal membranes. Exp Eye Res. 2009;88(3):438-44. doi: 10.1016/j.exer.2008.10.020 pmid: 19032953
Campochiaro PA, Hackett SF, Vinores SA, Freund J, Csaky C, LaRochelle W, et al. Platelet-derived growth factor is an autocrine growth stimulator in retinal pigmented epithelial cells. J Cell Sci. 1994;107 ( Pt 9):2459-69. pmid: 7844163
Campochiaro PA, Glaser BM. Platelet-derived growth factor is chemotactic for human retinal pigment epithelial cells. Arch Ophthalmol. 1985;103(4):576-9. doi: 10.1001/archopht.1985.01050040118034 pmid: 3985844
Harvey AK, Roberge F, Hjelmeland LM. Chemotaxis of rat retinal glia to growth factors found in repairing wounds. Invest Ophthalmol Vis Sci. 1987;28(7):1092-9. pmid: 3496317
Roldan-Pallares M, Rollin R, Martinez-Montero JC, Fernandez-Cruz A, Bravo-Llata C, Fernandez-Durango R. Immunoreactive endothelin-1 in the vitreous humor and epiretinal membranes of patients with proliferative diabetic retinopathy. Retina. 2007;27(2):222-35. doi: 10.1097/01.iae.0000231376.76601.40 pmid: 17290206
Snead MP, Snead DR, Richards AJ, Harrison JB, Poulson AV, Morris AH, et al. Clinical, histological and ultrastructural studies of the posterior hyaloid membrane. Eye (Lond). 2002;16(4):447-53. doi: 10.1038/sj.eye.6700198 pmid: 12101452
Mandal N, Kofod M, Vorum H, Villumsen J, Eriksen J, Heegaard S, et al. Proteomic analysis of human vitreous associated with idiopathic epiretinal membrane. Acta Ophthalmol. 2013;91(4):e333-4. doi: 10.1111/aos.12075 pmid: 23437965
Plekhanova OS, Stepanova VV, Ratner EI, Bobik A, Tkachuk VA, Parfyonova YV. Urokinase plasminogen activator in injured adventitia increases the number of myofibroblasts and augments early proliferation. J Vasc Res. 2006;43(5):437-46. doi: 10.1159/000094906 pmid: 16899994
Shi Q, Le X, Abbruzzese JL, Peng Z, Qian CN, Tang H, et al. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61(10):4143-54. pmid: 11358838
Widyantoro B, Emoto N, Nakayama K, Anggrahini DW, Adiarto S, Iwasa N, et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121(22):2407-18. doi: 10.1161/CIRCULATIONAHA.110.938217 pmid: 20497976
Tonnessen T, Giaid A, Saleh D, Naess PA, Yanagisawa M, Christensen G. Increased in vivo expression and production of endothelin-1 by porcine cardiomyocytes subjected to ischemia. Circ Res. 1995;76(5):767-72. doi: 10.1161/01.res.76.5.767 pmid: 7728993
Wang X, Guo Z, Ding Z, Khaidakov M, Lin J, Xu Z, et al. Endothelin-1 upregulation mediates aging-related cardiac fibrosis. J Mol Cell Cardiol. 2015;80:101-9. doi: 10.1016/j.yjmcc.2015.01.001 pmid: 25584774
Telander DG, Yu AK, Forward KI, Morales SA, Morse LS, Park SS, et al. Epithelial Membrane Protein-2 in Human Proliferative Vitreoretinopathy and Epiretinal Membranes. Invest Ophthalmol Vis Sci. 2016;57(7):3112-7. doi: 10.1167/iovs.15-17791 pmid: 27294805
Motulsky E, Salik D, Janssens X, Pion B, Dufrane R, Chaput F, et al. Aquaporin-1 expression in proliferative vitreoretinopathy and in epiretinal membranes. ScientificWorldJournal. 2014;2014:876208. doi: 10.1155/2014/876208 pmid: 24688444
Agre P. Nobel Lecture. Aquaporin water channels. Biosci Rep. 2004;24(3):127-63. doi: 10.1007/s10540-005-2577-2 pmid: 16209125
Vogler S, Pannicke T, Hollborn M, Grosche A, Busch S, Hoffmann S, et al. Muller cell reactivity in response to photoreceptor degeneration in rats with defective polycystin-2. PLoS One. 2014;8(6):e61631. doi: 10.1371/journal.pone.0061631 pmid: 23755094
Fukuda M, Nakanishi Y, Fuse M, Yokoi N, Hamada Y, Fukagawa M, et al. Altered expression of aquaporins 1 and 4 coincides with neurodegenerative events in retinas of spontaneously diabetic Torii rats. Exp Eye Res. 2010;90(1):17-25. doi: 10.1016/j.exer.2009.09.003 pmid: 19748503
Xun W, Liu Y, Qing G, Xun X, Dongqing Z, Haixiang W. Aquaporin 1 expression in retinal neovascularization in a mouse model of retinopathy of prematurity. Prep Biochem Biotechnol. 2009;39(2):208-17. doi: 10.1080/10826060902800882 pmid: 19291583
Iandiev I, Pannicke T, Reichel MB, Wiedemann P, Reichenbach A, Bringmann A. Expression of aquaporin-1 immunoreactivity by photoreceptor cells in the mouse retina. Neurosci Lett. 2005;388(2):96-9. doi: 10.1016/j.neulet.2005.06.046 pmid: 16039047
Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434(7034):786-92. doi: 10.1038/nature03460 pmid: 15815633
Hara-Chikuma M, Verkman AS. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med (Berl). 2008;86(2):221-31. doi: 10.1007/s00109-007-0272-4 pmid: 17968524
Hara-Chikuma M, Verkman AS. Aquaporin-1 facilitates epithelial cell migration in kidney proximal tubule. J Am Soc Nephrol. 2006;17(1):39-45. doi: 10.1681/ASN.2005080846 pmid: 16319186
McCoy E, Sontheimer H. Expression and function of water channels (aquaporins) in migrating malignant astrocytes. Glia. 2007;55(10):1034-43. doi: 10.1002/glia.20524 pmid: 17549682
Ruiz-Ederra J, Verkman AS. Aquaporin-1-facilitated keratocyte migration in cell culture and in vivo corneal wound healing models. Exp Eye Res. 2009;89(2):159-65. doi: 10.1016/j.exer.2009.03.002 pmid: 19298815
Hayashi S, Takahashi N, Kurata N, Yamaguchi A, Matsui H, Kato S, et al. Involvement of aquaporin-1 in gastric epithelial cell migration during wound repair. Biochem Biophys Res Commun. 2009;386(3):483-7. doi: 10.1016/j.bbrc.2009.06.067 pmid: 19539607
Monzani E, Bazzotti R, Perego C, La Porta CA. AQP1 is not only a water channel: it contributes to cell migration through Lin7/beta-catenin. PLoS One. 2009;4(7):e6167. doi: 10.1371/journal.pone.0006167 pmid: 19584911
Meng F, Rui Y, Xu L, Wan C, Jiang X, Li G. Aqp1 enhances migration of bone marrow mesenchymal stem cells through regulation of FAK and beta-catenin. Stem Cells Dev. 2014;23(1):66-75. doi: 10.1089/scd.2013.0185 pmid: 23962074
Levin ER, Frank HJ. Natriuretic peptides inhibit rat astroglial proliferation: mediation by C receptor. Am J Physiol. 1991;261(2 Pt 2):R453-7. doi: 10.1152/ajpregu.1991.261.2.R453 pmid: 1652217
Li H, Wang H, Wang F, Gu Q, Xu X. Snail involves in the transforming growth factor beta1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells. PLoS One. 2011;6(8):e23322. doi: 10.1371/journal.pone.0023322 pmid: 21853110
Bajorath J. Molecular organization, structural features, and ligand binding characteristics of CD44, a highly variable cell surface glycoprotein with multiple functions. Proteins. 2000;39(2):103-11. pmid: 10737932
Takahashi E, Nagano O, Ishimoto T, Yae T, Suzuki Y, Shinoda T, et al. Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction. J Biol Chem. 2010;285(6):4060-73. doi: 10.1074/jbc.M109.056523 pmid: 19965872
Distler JH, Schett G, Gay S, Distler O. The controversial role of tumor necrosis factor alpha in fibrotic diseases. Arthritis Rheum. 2008;58(8):2228-35. doi: 10.1002/art.23645 pmid: 18668576
Mia MM, Boersema M, Bank RA. Interleukin-1beta attenuates myofibroblast formation and extracellular matrix production in dermal and lung fibroblasts exposed to transforming growth factor-beta1. PLoS One. 2014;9(3):e91559. doi: 10.1371/journal.pone.0091559 pmid: 24622053
Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117(3):557-67. doi: 10.1172/JCI31139 pmid: 17332883
Sziksz E, Pap D, Lippai R, Beres NJ, Fekete A, Szabo AJ, et al. Fibrosis Related Inflammatory Mediators: Role of the IL-10 Cytokine Family. Mediators Inflamm. 2015;2015:764641. doi: 10.1155/2015/764641 pmid: 26199463
Brucher BL, Jamall IS. Epistemology of the origin of cancer: a new paradigm. BMC Cancer. 2014;14:331. doi: 10.1186/1471-2407-14-331 pmid: 24885752
Brucher BL, Jamall IS. Somatic Mutation Theory - Why it's Wrong for Most Cancers. Cell Physiol Biochem. 2016;38(5):1663-80. doi: 10.1159/000443106 pmid: 27160408
Bandapalli OR, Brücher BLDM, Jamall IS. Synopsis: Special Issue on “Disruption of signaling homeostasis induced crosstalk in the carcinogenesis paradigm Epistemology of the origin of cancerâ€. 4open. 2019;2. doi: 10.1051/fopen/2019023
- Abstract Viewed: 766 times
- Full Text PDF Downloaded: 408 times


