Systemic complications of intravitreal bevacizumab: a case report and literature review
Medical hypothesis, discovery & innovation in optometry,
Vol. 4 No. 2 (2023),
22 June 2023
,
Page 63-75
https://doi.org/10.51329/mehdioptometry175
Abstract
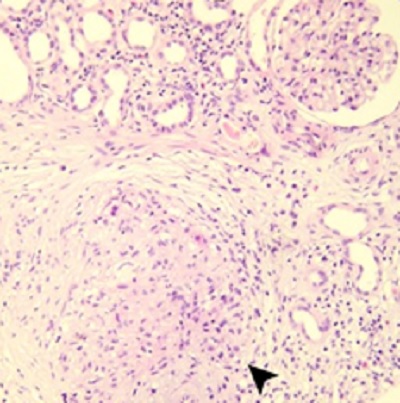
Background: Systemic complications of intravitreal bevacizumab (IVB) injections have been previously reported. We aimed to summarize the systemic complications reported in online primary studies. Moreover, we describe a patient experiencing simultaneous renal and cutaneous drug-induced adverse effects, with exacerbation of chronic renal insufficiency and granulomatous skin lesions, after receiving several IVB injections to manage bilateral ischemic branch retinal vein occlusion (BRVO).Case Presentation: A 69-year-old Hispanic diabetic man with chronic renal insufficiency due to polyclonal gammopathy received several IVB injections to treat bilateral ischemic BRVO. One week after the sixth injection, the patient developed acute-on-chronic renal failure and multiple rounded maculopapular, erythematous, and ulcerated skin lesions. Renal and skin biopsy specimens revealed granulomatous drug-induced responses in both organs, and granulomatous diseases of infectious and oncological sources were ruled out. We performed an electronic search of the PubMed/MEDLINE database with no language or time restrictions using the keywords “intravitreal bevacizumab” or “intravitreal Avastin” combined with “systemic side effects,” “systemic complications,” or “systemic adverse,” or “systemic adverse event.” The search yielded 147 articles published over almost two decades. After screening and assessment, we selected and summarized 40 primary studies that mentioned IVB-related systemic complications.
Conclusions: IVB-induced systemic complications, such as arteriothrombotic events, venous thrombotic events, and hypertension, are rare but potentially serious. Care should be taken when administering multiple doses of intravitreal IVB to patients with pre-existing kidney dysfunction. Bevacizumab-related toxicity must be considered in cases of sudden deterioration of renal function and / or unexpected granulomatous skin lesions in oncologic or chronically polymedicated patients.
- state of the art reviews
- case study
- avastin
- intravitreal injection
- chronic renal insufficiency
- drug side effect
- drug toxicity
References
Balci S, Sahin O, Ozcaliskan S, Sevik MO, Mangan MS. Immediate changes in blood pressure during intravitreal anti-VEGF agents' applications in exudative age-related macular degeneration patients. Int Ophthalmol. 2020;40(10):2515-2522. doi: 10.1007/s10792-020-01431-3 pmid: 32495059
Kodjikian L, Souied EH, Mimoun G, Mauget-Faÿsse M, Behar-Cohen F, Decullier E, et al; GEFAL Study Group. Ranibizumab versus Bevacizumab for Neovascular Age-related Macular Degeneration: Results from the GEFAL Noninferiority Randomized Trial. Ophthalmology. 2013;120(11):2300-9. doi: 10.1016/j.ophtha.2013.06.020 pmid: 23916488
Sharma S, Johnson D, Abouammoh M, Hollands S, Brissette A. Rate of serious adverse effects in a series of bevacizumab and ranibizumab injections. Can J Ophthalmol. 2012;47(3):275-9. doi: 10.1016/j.jcjo.2012.03.026 pmid: 22687306
di Lauro R, De Ruggiero P, di Lauro R, di Lauro MT, Romano MR. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248(6):785-91. doi: 10.1007/s00417-010-1303-3 pmid: 20135139
Bressler NM. Antiangiogenic approaches to age-related macular degeneration today. Ophthalmology. 2009;116(10 Suppl):S15-23. doi: 10.1016/j.ophtha.2009.06.048 pmid: 19800535
Nicholson BP, Schachat AP. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):915-30. doi: 10.1007/s00417-010-1315-z pmid: 20174816
Wong TY, Scott IU. Clinical practice. Retinal-vein occlusion. N Engl J Med. 2010;363(22):2135-44. doi: 10.1056/NEJMcp1003934 pmid: 21105795
Kameda Y, Babazono T, Uchigata Y, Kitano S. Renal function after intravitreal administration of vascular endothelial growth factor inhibitors in patients with diabetes and chronic kidney disease. J Diabetes Investig. 2018;9(4):937-939. doi: 10.1111/jdi.12771 pmid: 29108104
Wang X, Sawada T, Sawada O, Saishin Y, Liu P, Ohji M. Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am J Ophthalmol. 2014;158(4):738-744.e1. doi: 10.1016/j.ajo.2014.06.009 pmid: 24973606
Stewart MW, Rosenfeld PJ, Penha FM, Wang F, Yehoshua Z, Bueno-Lopez E, et al. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye). Retina. 2012;32(3):434-57. doi: 10.1097/IAE.0B013E31822C290F pmid: 22374154
Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol. 2014;98(12):1636-41. doi: 10.1136/bjophthalmol-2014-305252 pmid: 25001321
Ip MS, Scott IU, Brown GC, Brown MM, Ho AC, Huang SS, et al; American Academy of Ophthalmology. Anti-vascular endothelial growth factor pharmacotherapy for age-related macular degeneration: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115(10):1837-46. doi: 10.1016/j.ophtha.2008.08.012 pmid: 18929163
Schmucker C, Loke YK, Ehlken C, Agostini HT, Hansen LL, Antes G, et al. Intravitreal bevacizumab (Avastin) versus ranibizumab (Lucentis) for the treatment of age-related macular degeneration: a safety review. Br J Ophthalmol. 2011;95(3):308-17. doi: 10.1136/bjo.2009.178574 pmid: 20971791
Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group; Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388-98. doi: 10.1016/j.ophtha.2012.03.053 pmid: 22555112
Berg K, Pedersen TR, Sandvik L, Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015 ;122(1):146-52. doi: 10.1016/j.ophtha.2014.07.041 pmid: 25227499
Touzani F, Geers C, Pozdzik A. Intravitreal Injection of Anti-VEGF Antibody Induces Glomerular Endothelial Cells Injury. Case Rep Nephrol. 2019;2019:2919080. doi: 10.1155/2019/2919080 pmid: 31934470
Roth DB, King A, Weiss M, Klein D. Systemic adverse events after bevacizumab. Ophthalmology. 2009;116(6):1226.e1. doi: 10.1016/j.ophtha.2009.02.011 pmid: 19486800
Fam A, Finger PT. Cutaneous Cell-Mediated Delayed Hypersensitivity to Intravitreal Bevacizumab. Middle East Afr J Ophthalmol. 2020;27(3):182-184. doi: 10.4103/meajo.MEAJO_123_20 pmid: 33488016
Amselem L, Diaz-Llopis M, Garcia-Delpech S, Montero J, Palomares P, Cervera E. Papulopustular eruption after intravitreal bevacizumab (Avastin). Acta Ophthalmol. 2009;87(1):110-1. doi: 10.1111/j.1755-3768.2007.01154.x pmid: 18577190
Johari MK, Askari M, Amini A, Yasemi M. Acute systemic complications of intravitreal bevacizumab and triamcinolone injections - a comparative study. Folia Med (Plovdiv). 2022;64(2):240-247. doi: 10.3897/folmed.64.e61424 pmid: 35851775
Weinstein O, Abu Tailakh M, Lifshitz T, Novack V, Levy J. Intravitreal bevacizumab treatment for neovascular age-related macular degeneration and thromboembolic events. Eur J Ophthalmol. 2020;30(1):66-71. doi: 10.1177/1120672118823128 pmid: 30618282
Kunzmann S, Ngyuen T, Stahl A, Walz JM, Nentwich MM, Speer CP, et al. Necrotizing enterocolitis after intravitreal bevacizumab in an infant with Incontinentia Pigmenti - a case report. BMC Pediatr. 2019;19(1):353. doi: 10.1186/s12887-019-1732-z pmid: 31615465
Lekha T, Prasad HN, Sarwate RN, Patel M, Karthikeyan S. Intravitreal Bevacizumab for Choroidal Neovascularization Associated with Angioid Streaks: Long-term Results. Middle East Afr J Ophthalmol. 2017;24(3):136-142. doi: 10.4103/meajo.MEAJO_17_17 pmid: 29279654
Schauwvlieghe AM, Dijkman G, Hooymans JM, Verbraak FD, Hoyng CB, Dijkgraaf MG, et al. Comparing the Effectiveness of Bevacizumab to Ranibizumab in Patients with Exudative Age-Related Macular Degeneration. The BRAMD Study. PLoS One. 2016;11(5):e0153052. doi: 10.1371/journal.pone.0153052 pmid: 27203434
Krebs I, Schmetterer L, Boltz A, Told R, Vécsei-Marlovits V, Egger S, et al; MANTA Research Group. A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Ophthalmol. 2013;97(3):266-71. doi: 10.1136/bjophthalmol-2012-302391 pmid: 23292928
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258-67. doi: 10.1016/S0140-6736(13)61501-9 pmid: 23870813
Besozzi G, Ferrara A, Epifani E, Intini D, Apruzzese M, Provenzano A, et al. Acute stroke after intravitreal bevacizumab to treat choroidal neovascularization due to angioid streaks in pseudoxanthoma elasticum : a severe systemic adverse event after an off-label procedure. Int Ophthalmol. 2013;33(2):181-3. doi: 10.1007/s10792-012-9647-9 pmid: 23065017
IVAN Study Investigators; Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399-411. doi: 10.1016/j.ophtha.2012.04.015. Erratum in: Ophthalmology. 2012;119(8):1508. Erratum in: Ophthalmology. 2013;120(9):1719. pmid: 22578446
Micieli JA, Santiago P, Brent MH. Third nerve palsy following intravitreal anti-VEGF therapy for bilateral neovascular age-related macular degeneration. Acta Ophthalmol. 2011;89(1):e99-100. doi: 10.1111/j.1755-3768.2009.01778.x pmid: 19925526
CATT Research Group; Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-908. doi: 10.1056/NEJMoa1102673 pmid: 21526923
Luu ST, Gray T, Warrier SK, Patel I, Muecke JS, Casson R, et al. Retrospective study of an as required dosing regimen of intravitreal bevacizumab in neovascular age-related macular degeneration in an Australian population. Clin Exp Ophthalmol. 2010;38(7):659-63. doi: 10.1111/j.1442-9071.2010.02309.x pmid: 20456433
Yohendran J, Chauhan D. Erectile dysfunction following intravitreal bevacizumab. Middle East Afr J Ophthalmol. 2010;17(3):281-4. doi: 10.4103/0974-9233.65497 pmid: 20844689
Cakmak HB, Toklu Y, Yorgun MA, Sim?ek S. Isolated sixth nerve palsy after intravitreal bevacizumab injection. Strabismus. 2010;18(1):18-20. doi: 10.3109/09273970903567626 pmid: 20230202
Tufail A, Patel PJ, Egan C, Hykin P, da Cruz L, Gregor Z, et al; ABC Trial Investigators. Bevacizumab for neovascular age related macular degeneration (ABC Trial): multicentre randomised double masked study. BMJ. 2010;340:c2459. doi: 10.1136/bmj.c2459 pmid: 20538634
Petrou P, Georgalas I, Giavaras G, Anastasiou E, Ntana Z, Petrou C. Early loss of pregnancy after intravitreal bevacizumab injection. Acta Ophthalmol. 2010;88(4):e136. doi: 10.1111/j.1755-3768.2009.01572.x pmid: 19740128
Curtis LH, Hammill BG, Schulman KA, Cousins SW. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol. 2010;128(10):1273-9. doi: 10.1001/archophthalmol.2010.223. Erratum in: Arch Ophthalmol. 2010;128(12):1623. pmid: 20937996
Potter MJ, Claudio CC, Szabo SM. A randomised trial of bevacizumab and reduced light dose photodynamic therapy in age-related macular degeneration: the VIA study. Br J Ophthalmol. 2010;94(2):174-9. doi: 10.1136/bjo.2008.155531 pmid: 19520690
Byeon SH, Kwon OW, Lee SC. Transient global amnesia following intravitreal injection of bevacizumab. Acta Ophthalmol. 2009;87(5):570. doi: 10.1111/j.1755-3768.2008.01216.x pmid: 18537925
Kaiser PK; Registry of Visudyne AMD Therapy Writing Committee; Boyer DS, Garcia R, Hao Y, Hughes MS, Jabbour NM, Kaiser PK, et al. Verteporfin photodynamic therapy combined with intravitreal bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2009;116(4):747-55, 755.e1. doi: 10.1016/j.ophtha.2008.12.057 pmid: 19243834
Soheilian M, Ramezani A, Obudi A, Bijanzadeh B, Salehipour M, Yaseri M, et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology. 2009;116(6):1142-50. doi: 10.1016/j.ophtha.2009.01.011 pmid: 19376585
Fong KC, Kirkpatrick N, Mohamed Q, Johnston RL. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration using a variable frequency regimen in eyes with no previous treatment. Clin Exp Ophthalmol. 2008;36(8):748-55. doi: 10.1111/j.1442-9071.2008.01873.x pmid: 19128380
Wong LJ, Desai RU, Jain A, Feliciano D, Moshfeghi DM, Sanislo SR, et al. Surveillance for potential adverse events associated with the use of intravitreal bevacizumab for retinal and choroidal vascular disease. Retina. 2008;28(8):1151-8. doi: 10.1097/IAE.0b013e31817e100f pmid: 18685542
Wu L, Martínez-Castellanos MA, Quiroz-Mercado H, Arevalo JF, Berrocal MH, Farah ME, et al; Pan American Collaborative Retina Group (PACORES). Twelve-month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan-American Collaborative Retina Study Group (PACORES). Graefes Arch Clin Exp Ophthalmol. 2008;246(1):81-7. doi: 10.1007/s00417-007-0660-z pmid: 17674014
Shima C, Sakaguchi H, Gomi F, Kamei M, Ikuno Y, Oshima Y, et al. Complications in patients after intravitreal injection of bevacizumab. Acta Ophthalmol. 2008;86(4):372-6. doi: 10.1111/j.1600-0420.2007.01067.x pmid: 18028234
Diabetic Retinopathy Clinical Research Network; Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114(10):1860-7. doi: 10.1016/j.ophtha.2007.05.062 pmid: 17698196
Park HJ, Guy J. Sixth nerve palsy post intravitreal bevacizumab for AMD: a new possibly causal relationship and complication? Binocul Vis Strabismus Q. 2007;22(4):209. pmid: 18163895
Rodrigues EB, Shiroma H, Meyer CH, Maia M, Farah ME. Metrorrhagia after intravitreal injection of bevacizumab. Acta Ophthalmol Scand. 2007;85(8):915-6. doi: 10.1111/j.1600-0420.2007.00968.x pmid: 17645422
Moshfeghi AA, Rosenfeld PJ, Puliafito CA, Michels S, Marcus EN, Lenchus JD, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twenty-four-week results of an uncontrolled open-label clinical study. Ophthalmology. 2006;113(11):2002.e1-12. doi: 10.1016/j.ophtha.2006.05.070 pmid: 17027972
Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90(11):1344-9. doi: 10.1136/bjo.2006.099598 pmid: 16854824
Spaide RF, Laud K, Fine HF, Klancnik JM Jr, Meyerle CB, Yannuzzi LA, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006;26(4):383-90. doi: 10.1097/01.iae.0000238561.99283.0e pmid: 16603955
Pellé G, Shweke N, Duong Van Huyen JP, Tricot L, Hessaïne S, Frémeaux-Bacchi V, et al. Systemic and kidney toxicity of intraocular administration of vascular endothelial growth factor inhibitors. Am J Kidney Dis. 2011;57(5):756-9. doi: 10.1053/j.ajkd.2010.11.030 pmid: 21295897
Saif MW, Longo WL, Israel G. Correlation between rash and a positive drug response associated with bevacizumab in a patient with advanced colorectal cancer. Clin Colorectal Cancer. 2008;7(2):144-8. doi: 10.3816/CCC.2008.n.020 pmid: 18501075
Shye M, Hanna RM, Patel SS, Tram-Tran N, Hou J, Mccannel C, et al. Worsening proteinuria and renal function after intravitreal vascular endothelial growth factor blockade for diabetic proliferative retinopathy. Clin Kidney J. 2020;13(6):969-980. doi: 10.1093/ckj/sfaa049 pmid: 33391740
Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65(6):2003-17. doi: 10.1111/j.1523-1755.2004.00621.x pmid: 15149314
Bagheri S, Dormanesh B, Afarid M, Sagheb MM. Proteinuria and Renal Dysfunction after Intravitreal Injection of Bevacizumab in Patients with Diabetic Nephropathy: A Prospective Observational Study. Galen Med J. 2018;7:e1299. doi: 10.22086/gmj.v0i0.1299 pmid: 34466447
Borrego Utiel FJ, Luque Barona R, Pérez Del Barrio P, Borrego Hinojosa J, Ramírez Tortosa C. Acute Kidney Injury due to granulomatous interstitial nephritis induced by tramadol administration. Nefrologia (Engl Ed). 2018;38(2):227-228. English, Spanish. doi: 10.1016/j.nefro.2017.05.005 pmid: 29471961
Joss N, Morris S, Young B, Geddes C. Granulomatous interstitial nephritis. Clin J Am Soc Nephrol. 2007;2(2):222-30. doi: 10.2215/CJN.01790506 pmid: 17699417
Costagliola C, Agnifili L, Arcidiacono B, Duse S, Fasanella V, Mastropasqua R, et al. Systemic thromboembolic adverse events in patients treated with intravitreal anti-VEGF drugs for neovascular age-related macular degeneration. Expert Opin Biol Ther. 2012;12(10):1299-313. doi: 10.1517/14712598.2012.707176 pmid: 22866908
Das A, McGuire PG, Rangasamy S. Diabetic Macular Edema: Pathophysiology and Novel Therapeutic Targets. Ophthalmology. 2015;122(7):1375-94. doi: 10.1016/j.ophtha.2015.03.024 pmid: 25935789
Semeraro F, Morescalchi F, Duse S, Gambicorti E, Cancarini A, Costagliola C. Pharmacokinetic and Pharmacodynamic Properties of Anti-VEGF Drugs After Intravitreal Injection. Curr Drug Metab. 2015;16(7):572-84. doi: 10.2174/1389200216666151001120831 pmid: 26424177
Popescu V, Pricopie S, Totir M, Iancu R, Yasyn S, Alexandrescu C. Clinical use of Bevacizumab in treating refractory glaucoma. J Med Life. 2015;8(1):8-12. pmid: 25914729
Kazazi-Hyseni F, Beijnen JH, Schellens JH. Bevacizumab. Oncologist. 2010;15(8):819-25. doi: 10.1634/theoncologist.2009-0317 pmid: 20688807
- Abstract Viewed: 0 times
- Full Text PDF Downloaded: 0 times