Immediate effects of artificial tears with and without preservatives containing hyaluronic acid and carboxymethyl cellulose
Medical hypothesis, discovery & innovation in optometry,
Vol. 4 No. 3 (2023),
3 October 2023
,
Page 102-111
https://doi.org/10.51329/mehdioptometry179
Abstract
Background: Currently, hyaluronic acid (HA) and carboxymethyl cellulose sodium (CMC) are common polymers incorporated in artificial tears (ATs). The aim of the present study was to evaluate the immediate effect of preservative- and preservative-free HA- and CMC-containing ATs on tear-film parameters and determine patient preference after AT instillation.Methods: In this prospective, double-blind, randomized, comparative study, we assessed fluorescein tear break-up time (TBUT), bulbar redness, and tear ferning pattern (TFP) up to 60 min after the instillation of ATs with and without preservatives containing HA and CMC in the recruited participants. To test patient preference, each patient was administered with the Ora Calibra™ Ocular Discomfort and 4-Symptom Questionnaire (OOD4SQ; scale of 0–5) before and 60 min after the instillation of ATs. The selection of 14 descriptive words based the 11-point Ora Calibra™ Drop Comfort Scale (ODCS; scale of 0–10) was administered immediately after instillation of each AT to test the drop comfort score.
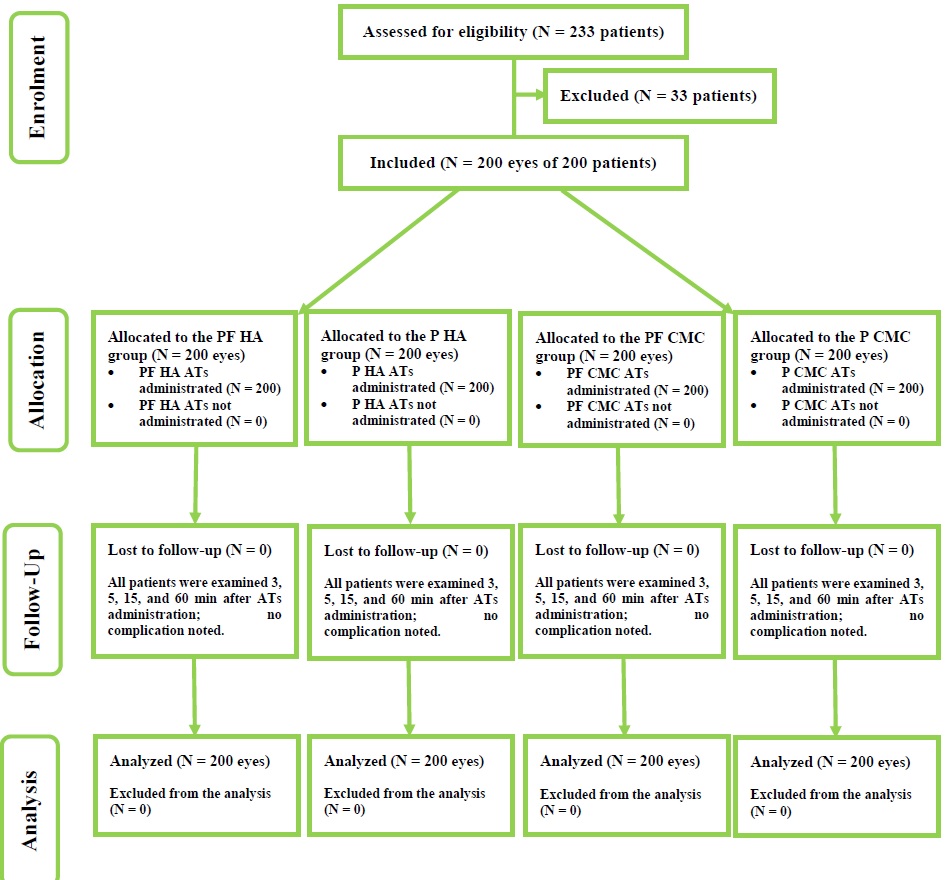
Results: We enrolled 200 eyes of 200 patients, including 163 (81.5%) women and 37 (18.5%) men, with a mean (standard deviation) age of 28.38 (5.42) years. Immediately or 5, 15, or 60 min after the instillation, the mean TBUT did not differ by presence of preservatives, HA, or CMC (all P > 0.05). However, it was significantly higher 5-min post-instillation compared to baseline and significantly lower 15- and 60-min post-instillation (all P < 0.05). The mean grade of bulbar redness immediately or 3, 5, 15, or 60 min after instillation did not differ by presence of preservatives for HA or CMC containing ATs (all P < 0.05). It did not differ significantly 3-, 5-, 15-, or 60-min post-instillation compared to baseline (all P > 0.05). The mean drop comfort scale after the instillation of ATs did not differ significantly by presence of preservatives, HA, or CMC (all P < 0.05). Positive descriptive words were selected by a higher proportion of participants in both groups. According to OOD4SQ, the overall discomfort and mean dryness scores improved significantly after instillation of HA-containing ATs (both P < 0.05), while the mean burning sensation, grittiness, and stinging scores remained unchanged (all P > 0.05). The overall discomfort and mean scores for each ocular symptom (P < 0.05), except for stinting (P > 0.05), improved significantly after instillation of CMC-containing ATs. The TFP did not change significantly from baseline to 60 min after the instillation of any AT (P > 0.05).
Conclusions: Both ATs with and without preservatives containing HA and CMC produced positive short-term objective and subjective effects. However, TBUT, TFP, bulbar redness, and patient feedback were comparable for both HA- and CMC-containing ATs. Further trials with longer observation periods or the recruitment of patients with different severities of dry eye could provide more robust and clinically applicable conclusions.
Keywords:
- hyaluronate sodium
- carboxymethyl cellulose
- tear
- patient preferences
- questionnaire
- artificial tear
References
1. Markoulli M, Sobbizadeh A, Tan J, Briggs N, Coroneo M. The Effect of Optive and Optive Advanced Artificial Tears on the Healthy Tear Film. Curr Eye Res. 2018;43(5):588-594. doi: 10.1080/02713683.2018.1433860 pmid: 29388845
2. Fogt JS, Kowalski MJ, King-Smith PE, Epitropolous AT, Hendershot AJ, Lembach C, et al. Tear lipid layer thickness with eye drops in meibomian gland dysfunction. Clin Ophthalmol. 2016;10:2237-2243. doi: 10.2147/OPTH.S120158 pmid: 27853352
3. Cwiklik L. Tear film lipid layer: A molecular level view. Biochim Biophys Acta. 2016;1858(10):2421-2430. doi: 10.1016/j.bbamem.2016.02.020 pmid: 26898663
4. Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66(2):190-194. doi: 10.4103/ijo.IJO_646_17 pmid: 29380756
5. Gipson IK. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2007;48(10):4390; 4391-8. doi: 10.1167/iovs.07-0770 pmid: 17898256
6. Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539-574. doi: 10.1016/j.jtos.2017.05.001 pmid: 28736342
7. Stonecipher KG, Torkildsen GL, Ousler GW 3rd, Morris S, Villanueva L, Hollander DA. The IMPACT study: a prospective evaluation of the effects of cyclosporine ophthalmic emulsion 0.05% on ocular surface staining and visual performance in patients with dry eye. Clin Ophthalmol. 2016;10:887-95. doi: 10.2147/OPTH.S101627 pmid: 27257373
8. Schmidl D, Schmetterer L, Witkowska KJ, Unterhuber A, dos Santos VA, Kaya S, et al. Tear film thickness after treatment with artificial tears in patients with moderate dry eye disease. Cornea. 2015;34(4):421-6. doi: 10.1097/ICO.0000000000000358 pmid: 25651494
9. Diaz-Llopis M, Pinazo-Duran MD, Diaz-Guiñon L, Rahhal-Ortuño M, Perez-Ramos M, Bosch R, et al. A randomized multicenter study comparing seawater washes and carmellose artificial tears eyedrops in the treatment of dry eye syndrome. Clin Ophthalmol. 2019;13:483-490. doi: 10.2147/OPTH.S185409 pmid: 30880909
10. Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312-34. doi: 10.1016/j.preteyeres.2010.03.001 pmid: 20302969
11. Stanton D, Batich C, Schultz G, Gibson D, Guidi C, Yang Q, et al. A Novel Method to Eliminate Preservatives in Eye Drops. J Ocul Pharmacol Ther. 2018;34(8):584-589. doi: 10.1089/jop.2018.0042 pmid: 30321108
12. Mantelli F, Tranchina L, Lambiase A, Bonini S. Ocular surface damage by ophthalmic compounds. Curr Opin Allergy Clin Immunol. 2011;11(5):464-70. doi: 10.1097/ACI.0b013e32834a95c9 pmid: 21822131
13. Aragona P, Benítez-Del-Castillo JM, Coroneo MT, Mukherji S, Tan J, Vandewalle E, et al. Safety and Efficacy of a Preservative-Free Artificial Tear Containing Carboxymethylcellulose and Hyaluronic Acid for Dry Eye Disease: A Randomized, Controlled, Multicenter 3-Month Study. Clin Ophthalmol. 2020;14:2951-2963. doi: 10.2147/OPTH.S256480 pmid: 33061281
14. Fallacara A, Vertuani S, Panozzo G, Pecorelli A, Valacchi G, Manfredini S. Novel Artificial Tears Containing Cross-Linked Hyaluronic Acid: An In Vitro Re-Epithelialization Study. Molecules. 2017;22(12):2104. doi: 10.3390/molecules22122104 pmid: 29189737
15. Bruix A, Adán A, Casaroli-Marano RP. Eficacia de la carboximetilcelulosa sódica para el tratamiento del síndrome del ojo seco [Efficacy of sodium carboxymethylcellulose in the treatment of dry eye syndrome]. Arch Soc Esp Oftalmol. 2006;81(2):85-92. Spanish. doi: 10.4321/s0365-66912006000200008 pmid: 16511715
16. Sanchez MA, Torralbo-Jimenez P, Giron N, de la Heras B, Herrero Vanrell R, Arriola-Villalobos P, et al. Comparative analysis of carmellose 0.5% versus hyaluronate 0.15% in dry eye: a flow cytometric study. Cornea. 2010;29(2):167-71. doi: 10.1097/ICO.0b013e3181b11648 pmid: 20023577
17. Hilmi MR, Che Azemin MZ, Mohd Kamal K, Mohd Tamrin MI, Abdul Gaffur N, Tengku Sembok TM. Prediction of Changes in Visual Acuity and Contrast Sensitivity Function by Tissue Redness after Pterygium Surgery. Curr Eye Res. 2017;42(6):852-856. doi: 10.1080/02713683.2016.1250277 pmid: 28118054
18. Alanazi SA, El-Hiti GA, Al-Baloud AA, Alfarhan MI, Al-Shahrani A, Albakri AA, et al. Effects of short-term oral vitamin A supplementation on the ocular tear film in patients with dry eye. Clin Ophthalmol. 2019;13:599-604. doi: 10.2147/OPTH.S198349 pmid: 31040640
19. Mohd RH, Che AM, Ithnin MH. Clinical Features of Lid Margin, Meibomian Gland and Tear Film Changes in Patients with Primary Pterygium. J Ophthalmic Res Ocular Care. 2022;5(1):92-6. doi: 10.36959/936/576
20. Ling TE, Othman K, Yan OP, Rashid RA, Tet CM, Yaakob A, et al. Evaluation of Ocular Surface Disease in Asian Patients with Primary Angle Closure. Open Ophthalmol J. 2017;11:31-39. doi: 10.2174/1874364101711010031 pmid: 28400889
21. Tsubota K, Yokoi N, Shimazaki J, Watanabe H, Dogru M, Yamada M, et al; Asia Dry Eye Society. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul Surf. 2017;15(1):65-76. doi: 10.1016/j.jtos.2016.09.003 pmid: 27725302
22. Tsubota K. Short Tear Film Breakup Time-Type Dry Eye. Invest Ophthalmol Vis Sci. 2018;59(14):DES64-DES70. doi: 10.1167/iovs.17-23746 pmid: 30481808
23. Köksoy Vay?so?lu S, Öncü E, Dursun Ö, Dinç E. Investigation of Dry Eye Symptoms in Lecturers by Ocular Surface Disease Index. Turk J Ophthalmol. 2019;49(3):142-148. doi: 10.4274/tjo.galenos.2018.67915 pmid: 31245976
24. Versura P, Fresina M, Campos EC. Ocular surface changes over the menstrual cycle in women with and without dry eye. Gynecol Endocrinol. 2007;23(7):385-90. doi: 10.1080/09513590701350390 pmid: 17701769
25. Gibson EJ, Stapleton F, Wolffsohn JS, Golebiowski B. Local synthesis of sex hormones: are there consequences for the ocular surface and dry eye? Br J Ophthalmol. 2017;101(12):1596-1603. doi: 10.1136/bjophthalmol-2017-310610 pmid: 28814411
26. Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71-81; quiz 82. doi: 10.3238/arztebl.2015.0071 pmid: 25686388
27. Foulks GN, Pflugfelder SC. New testing options for diagnosing and grading dry eye disease. Am J Ophthalmol. 2014;157(6):1122-9. doi: 10.1016/j.ajo.2014.03.002 pmid: 24631478
28. Mohd Radzi H, Khairidzan MK, Mohd Zulfaezal CA, Azrin EA. Corneo-pterygium total area measurements utilising image analysis method. J Optom. 2019;12(4):272-277. doi: 10.1016/j.optom.2019.04.001 pmid: 31097348
29. Efron N (2010). ‘Efron Grading Scales for Contact Lens Complications’. In Efron N (Ed.). Contact Lens Practice [Second edition] (pp. 459-462). Butterworth-Heinemann, Oxford. ISBN: 9780750688697. Link
30. Stein DM, Wollstein G, Ishikawa H, Hertzmark E, Noecker RJ, Schuman JS. Effect of corneal drying on optical coherence tomography. Ophthalmology. 2006;113(6):985-991. doi: 10.1016/j.ophtha.2006.02.018 pmid: 16751039
31. Horwath J, Ettinger K, Bachernegg M, Bodner E, Schmut O. Ocular Ferning test - effect of temperature and humidity on tear Ferning patterns. Ophthalmologica. 2001;215(2):102-7. doi: 10.1159/000050838 pmid: 11244339
32. Sharanjeet-Kaur, Ho CY, Mutalib HA, Ghazali AR. The Relationship Between Tear Ferning Patterns and Non-invasive Tear Break-up Time in Normal Asian Population. J Optom. 2016;9(3):175-81. doi: 10.1016/j.optom.2015.10.004 pmid: 26652245
33. Masmali AM. Improvement of ferning patterns of lubricant eye drops mixed with various electrolytes and carboxymethylcellulose. Cont Lens Anterior Eye. 2019;42(6):633-639. doi: 10.1016/j.clae.2019.04.010 pmid: 31010720
34. Torkildsen G, Brujic M, Cooper MS, Karpecki P, Majmudar P, Trattler W, et al. Evaluation of a new artificial tear formulation for the management of tear film stability and visual function in patients with dry eye. Clin Ophthalmol. 2017;11:1883-1889. doi: 10.2147/OPTH.S144369 pmid: 29089744
35. Che Arif FA, Hilmi MR, Kamal KM. A prospective contralateral eye comparison of the tolerability of two artificial tears with different physical properties in patients with dry eye disease. Med Hypothesis Discov Innov Optom. 2023; 4(1): 1-6. doi: 10.51329/mehdioptometry167
36. Ousler G 3rd, Devries DK, Karpecki PM, Ciolino JB. An evaluation of Retaine™ ophthalmic emulsion in the management of tear film stability and ocular surface staining in patients diagnosed with dry eye. Clin Ophthalmol. 2015;9:235-43. doi: 10.2147/OPTH.S75297 pmid: 25709384
37.Kaercher T, Buchholz P, Kimmich F. Treatment of patients with keratoconjunctivitis sicca with Optive: results of a multicenter, open-label observational study in Germany. Clin Ophthalmol. 2009;3:33-9 pmid: 19668542
38. Kathuria A, Shamloo K, Jhanji V, Sharma A. Categorization of Marketed Artificial Tear Formulations Based on Their Ingredients: A Rational Approach for Their Use. J Clin Med. 2021;10(6):1289. doi: 10.3390/jcm10061289 pmid: 33800965
39. Urbaniak GC, Plous S (2013).’Research Randomizer (Version 4.0) [Computer software]’. Available at: http://www.randomizer.org/ (Accessed: January 02, 2021)
40. Kaya S, Schmidl D, Schmetterer L, Witkowska KJ, Unterhuber A, Aranha Dos Santos V, et al. Effect of hyaluronic acid on tear film thickness as assessed with ultra-high resolution optical coherence tomography. Acta Ophthalmol. 2015;93(5):439-443. doi: 10.1111/aos.12647 pmid: 25601227
41. Yang YJ, Lee WY, Kim YJ, Hong YP. A Meta-Analysis of the Efficacy of Hyaluronic Acid Eye Drops for the Treatment of Dry Eye Syndrome. Int J Environ Res Public Health. 2021;18(5):2383. doi: 10.3390/ijerph18052383 pmid: 33804439
42. You IC, Li Y, Jin R, Ahn M, Choi W, Yoon KC. Comparison of 0.1%, 0.18%, and 0.3% Hyaluronic Acid Eye Drops in the Treatment of Experimental Dry Eye. J Ocul Pharmacol Ther. 2018;34(8):557-564. doi: 10.1089/jop.2018.0032 pmid: 30036099
43. Song JK, Lee K, Park HY, Hyon JY, Oh SW, Bae WK, et al. Efficacy of Carboxymethylcellulose and Hyaluronate in Dry Eye Disease: A Systematic Review and Meta-Analysis. Korean J Fam Med. 2017;38(1):2-7. doi: 10.4082/kjfm.2017.38.1.2 pmid: 28197326
44. Brignole F, Pisella PJ, Dupas B, Baeyens V, Baudouin C. Efficacy and safety of 0.18% sodium hyaluronate in patients with moderate dry eye syndrome and superficial keratitis. Graefes Arch Clin Exp Ophthalmol. 2005;243(6):531-8. doi: 10.1007/s00417-004-1040-6 pmid: 15965673
45. Groß D, Childs M, Piaton JM. Comparative study of 0.1% hyaluronic acid versus 0.5% carboxymethylcellulose in patients with dry eye associated with moderate keratitis or keratoconjunctivitis. Clin Ophthalmol. 2018;12:1081-1088. doi: 10.2147/OPTH.S161578 pmid: 29928109
46. Baudouin C, Cochener B, Pisella PJ, Girard B, Pouliquen P, Cooper H, et al. Randomized, phase III study comparing osmoprotective carboxymethylcellulose with sodium hyaluronate in dry eye disease. Eur J Ophthalmol. 2012;22(5):751-61. doi: 10.5301/ejo.5000117 pmid: 22287172
47. Lee JH, Ahn HS, Kim EK, Kim TI. Efficacy of sodium hyaluronate and carboxymethylcellulose in treating mild to moderate dry eye disease. Cornea. 2011;30(2):175-9. doi: 10.1097/ICO.0b013e3181e9adcc pmid: 21045674
48. Labetoulle M, Chiambaretta F, Shirlaw A, Leaback R, Baudouin C. Osmoprotectants, carboxymethylcellulose and hyaluronic acid multi-ingredient eye drop: a randomised controlled trial in moderate to severe dry eye. Eye (Lond). 2017;31(10):1512. doi: 10.1038/eye.2017.124. Erratum for: Eye (Lond). 2017;31(10 ):1409-1416 pmid: 29021564
49. Davitt WF, Bloomenstein M, Christensen M, Martin AE. Efficacy in patients with dry eye after treatment with a new lubricant eye drop formulation. J Ocul Pharmacol Ther. 2010;26(4):347-53. doi: 10.1089/jop.2010.0025 pmid: 20653478
50. Tavazzi S, Origgi R, Anselmi M, Corvino A, Colciago S, Fagnola M, et al. Effects of Aqueous-Supplementing Artificial Tears in Wearers of Biweekly Replacement Contact Lenses vs Wearers of Daily Disposable Contact Lenses. Clin Optom (Auckl). 2020;12:75-84. doi: 10.2147/OPTO.S249078 pmid: 32612403
51. Dutta D, Kim J, Sarkes M, Nath S, Markoulli M. The repeatability of subjective and objective tear ferning assessment and its association with lipid layer thickness, non-invasive tear break-up time and comfort. Cont Lens Anterior Eye. 2019;42(4):420-427. doi: 10.1016/j.clae.2019.04.003 pmid: 31029534
52. Alanazi SA, Badawood YS, Aldawood MA, El-Hiti GA, Masmali AM. Effect of Refresh Plus® preservative-free lubricant eyedrops on tear ferning patterns in dry eye and normal eye subjects. Clin Ophthalmol. 2019;13:1011-1017. doi: 10.2147/OPTH.S213365 pmid: 31354235
53. Masmali AM, Al-Bahlal JM, El-Hiti GA, Akhtar S, Purslow C, Murphy PJ, et al. Repeatability and Diurnal Variation of Tear Ferning Test. Eye Contact Lens. 2015;41(5):262-7. doi: 10.1097/ICL.0000000000000116 pmid: 25603440
54. Masmali AM, Al-Shehri A, Alanazi SA, Abusharaha A, Fagehi R, El-Hiti GA. Assessment of Tear Film Quality among Smokers Using Tear Ferning Patterns. J Ophthalmol. 2016;2016:8154315. doi: 10.1155/2016/8154315 pmid: 28003910
55. Masmali AM, Maeni YA, El-Hiti GA, Murphy PJ, Almubrad T. Investigation of Ocular Tear Ferning in Controlled and Uncontrolled Diabetic Subjects. Eye Contact Lens. 2018;44 Suppl 2:S70-S75. doi: 10.1097/ICL.0000000000000419 pmid: 28945647
56. Mencucci R, Boccalini C, Caputo R, Favuzza E. Effect of a hyaluronic acid and carboxymethylcellulose ophthalmic solution on ocular comfort and tear-film instability after cataract surgery. J Cataract Refract Surg. 2015;41(8):1699-704. doi: 10.1016/j.jcrs.2014.12.056 pmid: 26432128
57. Essa L, Laughton D, Wolffsohn JS. Can the optimum artificial tear treatment for dry eye disease be predicted from presenting signs and symptoms? Cont Lens Anterior Eye. 2018;41(1):60-68. doi: 10.1016/j.clae.2017.07.007 pmid: 28811095
2. Fogt JS, Kowalski MJ, King-Smith PE, Epitropolous AT, Hendershot AJ, Lembach C, et al. Tear lipid layer thickness with eye drops in meibomian gland dysfunction. Clin Ophthalmol. 2016;10:2237-2243. doi: 10.2147/OPTH.S120158 pmid: 27853352
3. Cwiklik L. Tear film lipid layer: A molecular level view. Biochim Biophys Acta. 2016;1858(10):2421-2430. doi: 10.1016/j.bbamem.2016.02.020 pmid: 26898663
4. Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66(2):190-194. doi: 10.4103/ijo.IJO_646_17 pmid: 29380756
5. Gipson IK. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2007;48(10):4390; 4391-8. doi: 10.1167/iovs.07-0770 pmid: 17898256
6. Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539-574. doi: 10.1016/j.jtos.2017.05.001 pmid: 28736342
7. Stonecipher KG, Torkildsen GL, Ousler GW 3rd, Morris S, Villanueva L, Hollander DA. The IMPACT study: a prospective evaluation of the effects of cyclosporine ophthalmic emulsion 0.05% on ocular surface staining and visual performance in patients with dry eye. Clin Ophthalmol. 2016;10:887-95. doi: 10.2147/OPTH.S101627 pmid: 27257373
8. Schmidl D, Schmetterer L, Witkowska KJ, Unterhuber A, dos Santos VA, Kaya S, et al. Tear film thickness after treatment with artificial tears in patients with moderate dry eye disease. Cornea. 2015;34(4):421-6. doi: 10.1097/ICO.0000000000000358 pmid: 25651494
9. Diaz-Llopis M, Pinazo-Duran MD, Diaz-Guiñon L, Rahhal-Ortuño M, Perez-Ramos M, Bosch R, et al. A randomized multicenter study comparing seawater washes and carmellose artificial tears eyedrops in the treatment of dry eye syndrome. Clin Ophthalmol. 2019;13:483-490. doi: 10.2147/OPTH.S185409 pmid: 30880909
10. Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312-34. doi: 10.1016/j.preteyeres.2010.03.001 pmid: 20302969
11. Stanton D, Batich C, Schultz G, Gibson D, Guidi C, Yang Q, et al. A Novel Method to Eliminate Preservatives in Eye Drops. J Ocul Pharmacol Ther. 2018;34(8):584-589. doi: 10.1089/jop.2018.0042 pmid: 30321108
12. Mantelli F, Tranchina L, Lambiase A, Bonini S. Ocular surface damage by ophthalmic compounds. Curr Opin Allergy Clin Immunol. 2011;11(5):464-70. doi: 10.1097/ACI.0b013e32834a95c9 pmid: 21822131
13. Aragona P, Benítez-Del-Castillo JM, Coroneo MT, Mukherji S, Tan J, Vandewalle E, et al. Safety and Efficacy of a Preservative-Free Artificial Tear Containing Carboxymethylcellulose and Hyaluronic Acid for Dry Eye Disease: A Randomized, Controlled, Multicenter 3-Month Study. Clin Ophthalmol. 2020;14:2951-2963. doi: 10.2147/OPTH.S256480 pmid: 33061281
14. Fallacara A, Vertuani S, Panozzo G, Pecorelli A, Valacchi G, Manfredini S. Novel Artificial Tears Containing Cross-Linked Hyaluronic Acid: An In Vitro Re-Epithelialization Study. Molecules. 2017;22(12):2104. doi: 10.3390/molecules22122104 pmid: 29189737
15. Bruix A, Adán A, Casaroli-Marano RP. Eficacia de la carboximetilcelulosa sódica para el tratamiento del síndrome del ojo seco [Efficacy of sodium carboxymethylcellulose in the treatment of dry eye syndrome]. Arch Soc Esp Oftalmol. 2006;81(2):85-92. Spanish. doi: 10.4321/s0365-66912006000200008 pmid: 16511715
16. Sanchez MA, Torralbo-Jimenez P, Giron N, de la Heras B, Herrero Vanrell R, Arriola-Villalobos P, et al. Comparative analysis of carmellose 0.5% versus hyaluronate 0.15% in dry eye: a flow cytometric study. Cornea. 2010;29(2):167-71. doi: 10.1097/ICO.0b013e3181b11648 pmid: 20023577
17. Hilmi MR, Che Azemin MZ, Mohd Kamal K, Mohd Tamrin MI, Abdul Gaffur N, Tengku Sembok TM. Prediction of Changes in Visual Acuity and Contrast Sensitivity Function by Tissue Redness after Pterygium Surgery. Curr Eye Res. 2017;42(6):852-856. doi: 10.1080/02713683.2016.1250277 pmid: 28118054
18. Alanazi SA, El-Hiti GA, Al-Baloud AA, Alfarhan MI, Al-Shahrani A, Albakri AA, et al. Effects of short-term oral vitamin A supplementation on the ocular tear film in patients with dry eye. Clin Ophthalmol. 2019;13:599-604. doi: 10.2147/OPTH.S198349 pmid: 31040640
19. Mohd RH, Che AM, Ithnin MH. Clinical Features of Lid Margin, Meibomian Gland and Tear Film Changes in Patients with Primary Pterygium. J Ophthalmic Res Ocular Care. 2022;5(1):92-6. doi: 10.36959/936/576
20. Ling TE, Othman K, Yan OP, Rashid RA, Tet CM, Yaakob A, et al. Evaluation of Ocular Surface Disease in Asian Patients with Primary Angle Closure. Open Ophthalmol J. 2017;11:31-39. doi: 10.2174/1874364101711010031 pmid: 28400889
21. Tsubota K, Yokoi N, Shimazaki J, Watanabe H, Dogru M, Yamada M, et al; Asia Dry Eye Society. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul Surf. 2017;15(1):65-76. doi: 10.1016/j.jtos.2016.09.003 pmid: 27725302
22. Tsubota K. Short Tear Film Breakup Time-Type Dry Eye. Invest Ophthalmol Vis Sci. 2018;59(14):DES64-DES70. doi: 10.1167/iovs.17-23746 pmid: 30481808
23. Köksoy Vay?so?lu S, Öncü E, Dursun Ö, Dinç E. Investigation of Dry Eye Symptoms in Lecturers by Ocular Surface Disease Index. Turk J Ophthalmol. 2019;49(3):142-148. doi: 10.4274/tjo.galenos.2018.67915 pmid: 31245976
24. Versura P, Fresina M, Campos EC. Ocular surface changes over the menstrual cycle in women with and without dry eye. Gynecol Endocrinol. 2007;23(7):385-90. doi: 10.1080/09513590701350390 pmid: 17701769
25. Gibson EJ, Stapleton F, Wolffsohn JS, Golebiowski B. Local synthesis of sex hormones: are there consequences for the ocular surface and dry eye? Br J Ophthalmol. 2017;101(12):1596-1603. doi: 10.1136/bjophthalmol-2017-310610 pmid: 28814411
26. Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71-81; quiz 82. doi: 10.3238/arztebl.2015.0071 pmid: 25686388
27. Foulks GN, Pflugfelder SC. New testing options for diagnosing and grading dry eye disease. Am J Ophthalmol. 2014;157(6):1122-9. doi: 10.1016/j.ajo.2014.03.002 pmid: 24631478
28. Mohd Radzi H, Khairidzan MK, Mohd Zulfaezal CA, Azrin EA. Corneo-pterygium total area measurements utilising image analysis method. J Optom. 2019;12(4):272-277. doi: 10.1016/j.optom.2019.04.001 pmid: 31097348
29. Efron N (2010). ‘Efron Grading Scales for Contact Lens Complications’. In Efron N (Ed.). Contact Lens Practice [Second edition] (pp. 459-462). Butterworth-Heinemann, Oxford. ISBN: 9780750688697. Link
30. Stein DM, Wollstein G, Ishikawa H, Hertzmark E, Noecker RJ, Schuman JS. Effect of corneal drying on optical coherence tomography. Ophthalmology. 2006;113(6):985-991. doi: 10.1016/j.ophtha.2006.02.018 pmid: 16751039
31. Horwath J, Ettinger K, Bachernegg M, Bodner E, Schmut O. Ocular Ferning test - effect of temperature and humidity on tear Ferning patterns. Ophthalmologica. 2001;215(2):102-7. doi: 10.1159/000050838 pmid: 11244339
32. Sharanjeet-Kaur, Ho CY, Mutalib HA, Ghazali AR. The Relationship Between Tear Ferning Patterns and Non-invasive Tear Break-up Time in Normal Asian Population. J Optom. 2016;9(3):175-81. doi: 10.1016/j.optom.2015.10.004 pmid: 26652245
33. Masmali AM. Improvement of ferning patterns of lubricant eye drops mixed with various electrolytes and carboxymethylcellulose. Cont Lens Anterior Eye. 2019;42(6):633-639. doi: 10.1016/j.clae.2019.04.010 pmid: 31010720
34. Torkildsen G, Brujic M, Cooper MS, Karpecki P, Majmudar P, Trattler W, et al. Evaluation of a new artificial tear formulation for the management of tear film stability and visual function in patients with dry eye. Clin Ophthalmol. 2017;11:1883-1889. doi: 10.2147/OPTH.S144369 pmid: 29089744
35. Che Arif FA, Hilmi MR, Kamal KM. A prospective contralateral eye comparison of the tolerability of two artificial tears with different physical properties in patients with dry eye disease. Med Hypothesis Discov Innov Optom. 2023; 4(1): 1-6. doi: 10.51329/mehdioptometry167
36. Ousler G 3rd, Devries DK, Karpecki PM, Ciolino JB. An evaluation of Retaine™ ophthalmic emulsion in the management of tear film stability and ocular surface staining in patients diagnosed with dry eye. Clin Ophthalmol. 2015;9:235-43. doi: 10.2147/OPTH.S75297 pmid: 25709384
37.Kaercher T, Buchholz P, Kimmich F. Treatment of patients with keratoconjunctivitis sicca with Optive: results of a multicenter, open-label observational study in Germany. Clin Ophthalmol. 2009;3:33-9 pmid: 19668542
38. Kathuria A, Shamloo K, Jhanji V, Sharma A. Categorization of Marketed Artificial Tear Formulations Based on Their Ingredients: A Rational Approach for Their Use. J Clin Med. 2021;10(6):1289. doi: 10.3390/jcm10061289 pmid: 33800965
39. Urbaniak GC, Plous S (2013).’Research Randomizer (Version 4.0) [Computer software]’. Available at: http://www.randomizer.org/ (Accessed: January 02, 2021)
40. Kaya S, Schmidl D, Schmetterer L, Witkowska KJ, Unterhuber A, Aranha Dos Santos V, et al. Effect of hyaluronic acid on tear film thickness as assessed with ultra-high resolution optical coherence tomography. Acta Ophthalmol. 2015;93(5):439-443. doi: 10.1111/aos.12647 pmid: 25601227
41. Yang YJ, Lee WY, Kim YJ, Hong YP. A Meta-Analysis of the Efficacy of Hyaluronic Acid Eye Drops for the Treatment of Dry Eye Syndrome. Int J Environ Res Public Health. 2021;18(5):2383. doi: 10.3390/ijerph18052383 pmid: 33804439
42. You IC, Li Y, Jin R, Ahn M, Choi W, Yoon KC. Comparison of 0.1%, 0.18%, and 0.3% Hyaluronic Acid Eye Drops in the Treatment of Experimental Dry Eye. J Ocul Pharmacol Ther. 2018;34(8):557-564. doi: 10.1089/jop.2018.0032 pmid: 30036099
43. Song JK, Lee K, Park HY, Hyon JY, Oh SW, Bae WK, et al. Efficacy of Carboxymethylcellulose and Hyaluronate in Dry Eye Disease: A Systematic Review and Meta-Analysis. Korean J Fam Med. 2017;38(1):2-7. doi: 10.4082/kjfm.2017.38.1.2 pmid: 28197326
44. Brignole F, Pisella PJ, Dupas B, Baeyens V, Baudouin C. Efficacy and safety of 0.18% sodium hyaluronate in patients with moderate dry eye syndrome and superficial keratitis. Graefes Arch Clin Exp Ophthalmol. 2005;243(6):531-8. doi: 10.1007/s00417-004-1040-6 pmid: 15965673
45. Groß D, Childs M, Piaton JM. Comparative study of 0.1% hyaluronic acid versus 0.5% carboxymethylcellulose in patients with dry eye associated with moderate keratitis or keratoconjunctivitis. Clin Ophthalmol. 2018;12:1081-1088. doi: 10.2147/OPTH.S161578 pmid: 29928109
46. Baudouin C, Cochener B, Pisella PJ, Girard B, Pouliquen P, Cooper H, et al. Randomized, phase III study comparing osmoprotective carboxymethylcellulose with sodium hyaluronate in dry eye disease. Eur J Ophthalmol. 2012;22(5):751-61. doi: 10.5301/ejo.5000117 pmid: 22287172
47. Lee JH, Ahn HS, Kim EK, Kim TI. Efficacy of sodium hyaluronate and carboxymethylcellulose in treating mild to moderate dry eye disease. Cornea. 2011;30(2):175-9. doi: 10.1097/ICO.0b013e3181e9adcc pmid: 21045674
48. Labetoulle M, Chiambaretta F, Shirlaw A, Leaback R, Baudouin C. Osmoprotectants, carboxymethylcellulose and hyaluronic acid multi-ingredient eye drop: a randomised controlled trial in moderate to severe dry eye. Eye (Lond). 2017;31(10):1512. doi: 10.1038/eye.2017.124. Erratum for: Eye (Lond). 2017;31(10 ):1409-1416 pmid: 29021564
49. Davitt WF, Bloomenstein M, Christensen M, Martin AE. Efficacy in patients with dry eye after treatment with a new lubricant eye drop formulation. J Ocul Pharmacol Ther. 2010;26(4):347-53. doi: 10.1089/jop.2010.0025 pmid: 20653478
50. Tavazzi S, Origgi R, Anselmi M, Corvino A, Colciago S, Fagnola M, et al. Effects of Aqueous-Supplementing Artificial Tears in Wearers of Biweekly Replacement Contact Lenses vs Wearers of Daily Disposable Contact Lenses. Clin Optom (Auckl). 2020;12:75-84. doi: 10.2147/OPTO.S249078 pmid: 32612403
51. Dutta D, Kim J, Sarkes M, Nath S, Markoulli M. The repeatability of subjective and objective tear ferning assessment and its association with lipid layer thickness, non-invasive tear break-up time and comfort. Cont Lens Anterior Eye. 2019;42(4):420-427. doi: 10.1016/j.clae.2019.04.003 pmid: 31029534
52. Alanazi SA, Badawood YS, Aldawood MA, El-Hiti GA, Masmali AM. Effect of Refresh Plus® preservative-free lubricant eyedrops on tear ferning patterns in dry eye and normal eye subjects. Clin Ophthalmol. 2019;13:1011-1017. doi: 10.2147/OPTH.S213365 pmid: 31354235
53. Masmali AM, Al-Bahlal JM, El-Hiti GA, Akhtar S, Purslow C, Murphy PJ, et al. Repeatability and Diurnal Variation of Tear Ferning Test. Eye Contact Lens. 2015;41(5):262-7. doi: 10.1097/ICL.0000000000000116 pmid: 25603440
54. Masmali AM, Al-Shehri A, Alanazi SA, Abusharaha A, Fagehi R, El-Hiti GA. Assessment of Tear Film Quality among Smokers Using Tear Ferning Patterns. J Ophthalmol. 2016;2016:8154315. doi: 10.1155/2016/8154315 pmid: 28003910
55. Masmali AM, Maeni YA, El-Hiti GA, Murphy PJ, Almubrad T. Investigation of Ocular Tear Ferning in Controlled and Uncontrolled Diabetic Subjects. Eye Contact Lens. 2018;44 Suppl 2:S70-S75. doi: 10.1097/ICL.0000000000000419 pmid: 28945647
56. Mencucci R, Boccalini C, Caputo R, Favuzza E. Effect of a hyaluronic acid and carboxymethylcellulose ophthalmic solution on ocular comfort and tear-film instability after cataract surgery. J Cataract Refract Surg. 2015;41(8):1699-704. doi: 10.1016/j.jcrs.2014.12.056 pmid: 26432128
57. Essa L, Laughton D, Wolffsohn JS. Can the optimum artificial tear treatment for dry eye disease be predicted from presenting signs and symptoms? Cont Lens Anterior Eye. 2018;41(1):60-68. doi: 10.1016/j.clae.2017.07.007 pmid: 28811095
- Abstract Viewed: 0 times
- Full Text PDF Downloaded: 0 times