Persistent Corneal Epithelial Defects: A Review Article
Medical hypothesis discovery and innovation in ophthalmology,
Vol. 8 No. 3 (2019),
20 September 2019
,
Page 163-176
Abstract
Persistent corneal epithelial defects (PEDs or PCEDs) result from the failure of rapid re-epithelialization and closure within 10-14 days after a corneal injury, even with standard supportive treatment. Disruptions in the protective epithelial and stromal layers of the cornea can render the eye susceptible to infection, stromal ulceration, perforation, scarring, and significant vision loss. Although several therapies exist and an increasing number of novel approaches are emerging, treatment of PEDs can still be quite challenging. It is important to treat the underlying causative condition, which may include an infection, limbal stem cell deficiency, or diabetes, in order to facilitate wound healing. Standard treatments, such as bandage contact lenses (BCLs) and artificial tears (ATs), aim to provide barrier protection to the epithelial layer. Recently-developed medical treatments can target the re-epithelialization process by facilitating access to growth factors and anti-inflammatory agents, and novel surgical techniques can provide re-innervation to the cornea. PEDs should be treated within 7-10 days to avoid secondary complications. These interventions, along with a step-wise approach to management, can be useful in patients with PEDs that are refractory to standard medical treatment. In this review, we discuss the epidemiology, etiology, diagnosis, current and novel management, and prognosis of persistent epithelial defects.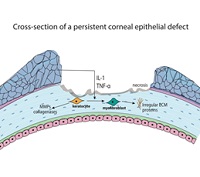
References
Eghrari AO, Riazuddin SA, Gottsch JD. Overview of the Cornea: Structure, Function, and Development. Prog Mol Biol Transl Sci. 2015;134:7-23. doi: 10.1016/bs.pmbts.2015.04.001 pmid: 26310146
Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood). 2001;226(7):653-64. doi: 10.1177/153537020222600711 pmid: 11444101
Katzman LR, Jeng BH. Management strategies for persistent epithelial defects of the cornea. Saudi J Ophthalmol. 2014;28(3):168-72. doi: 10.1016/j.sjopt.2014.06.011 pmid: 25278792
Dahlgren MA, Dhaliwal A, Huang AJW. Persistent Epithelial Defects. 2008:749-59. doi: 10.1016/b978-1-4160-0016-7.50058-8
Wilson SE, Medeiros CS, Santhiago MR. Pathophysiology of Corneal Scarring in Persistent Epithelial Defects After PRK and Other Corneal Injuries. J Refract Surg. 2018;34(1):59-64. doi: 10.3928/1081597X-20171128-01 pmid: 29315443
Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015;49:17-45. doi: 10.1016/j.preteyeres.2015.07.002 pmid: 26197361
Wirostko B, Rafii M, Sullivan DA, Morelli J, Ding J. Novel Therapy to Treat Corneal Epithelial Defects: A Hypothesis with Growth Hormone. Ocul Surf. 2015;13(3):204-12 e1. doi: 10.1016/j.jtos.2014.12.005 pmid: 26045234
Wan S, Cheng J, Dong Y, Xie L. Epithelial defects after penetrating keratoplasty in infectious keratitis: An analysis of characteristics and risk factors. PLoS One. 2018;13(11):e0208163. doi: 10.1371/journal.pone.0208163 pmid: 30485371
Germano EM, Mello MJ, Sena DF, Correia JB, Amorim MM. Incidence and risk factors of corneal epithelial defects in mechanically ventilated children. Crit Care Med. 2009;37(3):1097-100. doi: 10.1097/CCM.0b013e318196227d pmid: 19237923
Chen HF, Yeung L, Yang KJ, Sun CC. Persistent Corneal Epithelial Defect after Pars Plana Vitrectomy. Retina. 2016;36(1):148-55. doi: 10.1097/IAE.0000000000000657 pmid: 26166798
Griffith GL, Kasus-Jacobi A, Pereira HA. Bioactive Antimicrobial Peptides as Therapeutics for Corneal Wounds and Infections. Adv Wound Care (New Rochelle). 2017;6(6):175-90. doi: 10.1089/wound.2016.0713 pmid: 28616359
Chaurasia SS, Lim RR, Lakshminarayanan R, Mohan RR. Nanomedicine approaches for corneal diseases. J Funct Biomater. 2015;6(2):277-98. doi: 10.3390/jfb6020277 pmid: 25941990
Sangwan VS. Limbal stem cells in health and disease. Biosci Rep. 2001;21(4):385-405. doi: 10.1023/a:1017935624867 pmid: 11900318
Ahmad S. Concise review: limbal stem cell deficiency, dysfunction, and distress. Stem Cells Transl Med. 2012;1(2):110-5. doi: 10.5966/sctm.2011-0037 pmid: 23197757
Fatima A, Iftekhar G, Sangwan VS, Vemuganti GK. Ocular surface changes in limbal stem cell deficiency caused by chemical injury: a histologic study of excised pannus from recipients of cultured corneal epithelium. Eye (Lond). 2008;22(9):1161-7. doi: 10.1038/sj.eye.6702895 pmid: 17558385
Sacchetti M, Lambiase A. Neurotrophic factors and corneal nerve regeneration. Neural Regen Res. 2017;12(8):1220-4. doi: 10.4103/1673-5374.213534 pmid: 28966630
Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye (Lond). 2003;17(8):989-95. doi: 10.1038/sj.eye.6700616 pmid: 14631406
Singh P, Tyagi M, Kumar Y, Gupta KK, Sharma PD. Ocular chemical injuries and their management. Oman J Ophthalmol. 2013;6(2):83-6. doi: 10.4103/0974-620X.116624 pmid: 24082664
Ahmed F, House RJ, Feldman BH. Corneal Abrasions and Corneal Foreign Bodies. Prim Care. 2015;42(3):363-75. doi: 10.1016/j.pop.2015.05.004 pmid: 26319343
Sejpal K, Bakhtiari P, Deng SX. Presentation, diagnosis and management of limbal stem cell deficiency. Middle East Afr J Ophthalmol. 2013;20(1):5-10. doi: 10.4103/0974-9233.106381 pmid: 23580847
Fu Y, Liu J, Tseng SC. Ocular surface deficits contributing to persistent epithelial defect after penetrating keratoplasty. Cornea. 2012;31(7):723-9. doi: 10.1097/ICO.0b013e31821142ee pmid: 22495035
Petroutsos G, Guimaraes R, Giraud J, Pouliquen Y. Antibiotics and corneal epithelial wound healing. Arch Ophthalmol. 1983;101(11):1775-8. doi: 10.1001/archopht.1983.01040020777023 pmid: 6639435
Tuli SS, Schultz GS, Downer DM. Science and Strategy for Preventing and Managing Corneal Ulceration. The Ocular Surface. 2007;5(1):23-39. doi: 10.1016/s1542-0124(12)70050-2
Feizi S, Masoudi A, Hosseini SB, Kanavi MR, Javadi MA. Microbiological Evaluation of Bandage Soft Contact Lenses Used in Management of Persistent Corneal Epithelial Defects. Cornea. 2019;38(2):146-50. doi: 10.1097/ICO.0000000000001810 pmid: 30422865
Campanile TM, St Clair DA, Benaim M. The evaluation of eye patching in the treatment of traumatic corneal epithelial defects. J Emerg Med. 1997;15(6):769-74. doi: 10.1016/s0736-4679(97)00182-0 pmid: 9404791
Kitchens J, Kinder J, Oetting T. The drawstring temporary tarsorrhaphy technique. Arch Ophthalmol. 2002;120(2):187-90. doi: 10.1001/archopht.120.2.187 pmid: 11831921
Vote BJ, Elder MJ. Cyanoacrylate glue for corneal perforations: a description of a surgical technique and a review of the literature. Clin Exp Ophthalmol. 2000;28(6):437-42. pmid: 11202468
Perry HD, Kenyon KR, Lamberts DW, Foulks GN, Seedor JA, Golub LM. Systemic Tetracycline Hydrochloride as Adjunctive Therapy in the Treatment of Persistent Epithelial Defects. Ophthalmology. 1986;93(10):1320-2. doi: 10.1016/s0161-6420(86)33570-x
Hossain P. The corneal melting point. Eye (Lond). 2012;26(8):1029-30. doi: 10.1038/eye.2012.136 pmid: 22766538
Malhotra C, Jain AK. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J Transplant. 2014;4(2):111-21. doi: 10.5500/wjt.v4.i2.111 pmid: 25032100
Azuara-Blanco A, Pillai CT, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol. 1999;83(4):399-402. doi: 10.1136/bjo.83.4.399 pmid: 10434859
Lee SH, Tseng SC. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997;123(3):303-12. doi: 10.1016/s0002-9394(14)70125-4 pmid: 9063239
Prabhasawat P, Tesavibul N, Komolsuradej W. Single and multilayer amniotic membrane transplantation for persistent corneal epithelial defect with and without stromal thinning and perforation. Br J Ophthalmol. 2001;85(12):1455-63. doi: 10.1136/bjo.85.12.1455 pmid: 11734521
Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017;18(2):193-204. doi: 10.1007/s10561-017-9618-5 pmid: 28255771
Sabater AL, Perez VL. Amniotic membrane use for management of corneal limbal stem cell deficiency. Curr Opin Ophthalmol. 2017;28(4):363-9. doi: 10.1097/ICU.0000000000000386 pmid: 28426442
Tseng SC, Prabhasawat P, Barton K, Gray T, Meller D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol. 1998;116(4):431-41. doi: 10.1001/archopht.116.4.431 pmid: 9565039
Rahman I, Said DG, Maharajan VS, Dua HS. Amniotic membrane in ophthalmology: indications and limitations. Eye (Lond). 2009;23(10):1954-61. doi: 10.1038/eye.2008.410 pmid: 19169225
Lee YK, Lin YC, Tsai SH, Chen WL, Chen YM. Therapeutic outcomes of combined topical autologous serum eye drops with silicone-hydrogel soft contact lenses in the treatment of corneal persistent epithelial defects: A preliminary study. Cont Lens Anterior Eye. 2016;39(6):425-30. doi: 10.1016/j.clae.2016.06.003 pmid: 27349951
Wang WY, Lee YK, Tsai SH, Lin YC, Chen YM. Autologous Serum Eye Drops Combined With Silicone Hydrogen Lenses for the Treatment of Postinfectious Corneal Persistent Epithelial Defects. Eye Contact Lens. 2017;43(4):225-9. doi: 10.1097/ICL.0000000000000261 pmid: 26963437
Young AL, Cheng AC, Ng HK, Cheng LL, Leung GY, Lam DS. The use of autologous serum tears in persistent corneal epithelial defects. Eye (Lond). 2004;18(6):609-14. doi: 10.1038/sj.eye.6700721 pmid: 15184926
Jeng BH, Dupps WJ, Jr. Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea. 2009;28(10):1104-8. doi: 10.1097/ICO.0b013e3181a2a7f6 pmid: 19730088
Noble BA, Loh RS, MacLennan S, Pesudovs K, Reynolds A, Bridges LR, et al. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004;88(5):647-52. doi: 10.1136/bjo.2003.026211 pmid: 15090417
Schrader S, Wedel T, Moll R, Geerling G. Combination of serum eye drops with hydrogel bandage contact lenses in the treatment of persistent epithelial defects. Graefes Arch Clin Exp Ophthalmol. 2006;244(10):1345-9. doi: 10.1007/s00417-006-0257-y pmid: 16544115
Choi JA, Chung SH. Combined application of autologous serum eye drops and silicone hydrogel lenses for the treatment of persistent epithelial defects. Eye Contact Lens. 2011;37(6):370-3. doi: 10.1097/ICL.0b013e318233c9bb pmid: 21983552
Vajpayee RB, Mukerji N, Tandon R, Sharma N, Pandey RM, Biswas NR, et al. Evaluation of umbilical cord serum therapy for persistent corneal epithelial defects. Br J Ophthalmol. 2003;87(11):1312-6. doi: 10.1136/bjo.87.11.1312 pmid: 14609821
Soni NG, Jeng BH. Blood-derived topical therapy for ocular surface diseases. Br J Ophthalmol. 2016;100(1):22-7. doi: 10.1136/bjophthalmol-2015-306842 pmid: 26178904
Rosenthal P, Cotter JM, Baum J. Treatment of persistent corneal epithelial defect with extended wear of a fluid-ventilated gas-permeable scleral contact lens. Am J Ophthalmol. 2000;130(1):33-41. doi: 10.1016/s0002-9394(00)00379-2 pmid: 11004257
Lim P, Ridges R, Jacobs DS, Rosenthal P. Treatment of persistent corneal epithelial defect with overnight wear of a prosthetic device for the ocular surface. Am J Ophthalmol. 2013;156(6):1095-101. doi: 10.1016/j.ajo.2013.06.006 pmid: 24075432
Tsubota K, Satake Y, Kaido M, Shinozaki N, Shimmura S, Bissen-Miyajima H, et al. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340(22):1697-703. doi: 10.1056/NEJM199906033402201 pmid: 10352161
Koizumi N, Inatomi T, Suzuki T, Sotozono C, Kinoshita S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders11The authors have no financial interest in this work. Ophthalmology. 2001;108(9):1569-74. doi: 10.1016/s0161-6420(01)00694-7
Vajpayee RB, Thomas S, Sharma N, Dada T, Tabin GC. Large-diameter lamellar keratoplasty in severe ocular alkali burns. Ophthalmology. 2000;107(9):1765-8. doi: 10.1016/s0161-6420(00)00250-5
Sejpal K, Yu F, Aldave AJ. The Boston keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea. 2011;30(11):1187-94. doi: 10.1097/ICO.0b013e3182114467 pmid: 21885964
Kim MS, Song SW, Kim JH, Woo HM. Multifocal phototherapeutic keratectomy for the treatment of persistent epithelial defect. J Cataract Refract Surg. 2000;26(12):1753-7. doi: 10.1016/s0886-3350(00)00640-4 pmid: 11134875
Rathi VM, Vyas SP, Sangwan VS. Phototherapeutic keratectomy. Indian J Ophthalmol. 2012;60(1):5-14. doi: 10.4103/0301-4738.91335 pmid: 22218239
Nishida T, Ohashi Y, Awata T, Manabe R. Fibronectin. A new therapy for corneal trophic ulcer. Arch Ophthalmol. 1983;101(7):1046-8. doi: 10.1001/archopht.1983.01040020048007 pmid: 6870626
McCulley JP, Horowitz B, Husseini ZM, Horowitz M. Topical fibronectin therapy of persistent corneal epithelial defects. Fibronectin Study Group. Trans Am Ophthalmol Soc. 1993;91:367-86; discussion 86-90. pmid: 8140699
Spigelman AV, Deutsch TA, Sugar J. Application of homologous fibronectin to persistent human corneal epithelial defects. Cornea. 1987;6(2):128-30. pmid: 3608513
Gordon JF, Johnson P, Musch DC. Topical fibronectin ophthalmic solution in the treatment of persistent defects of the corneal epithelium. Chiron Vision Fibronectin Study Group. Am J Ophthalmol. 1995;119(3):281-7. doi: 10.1016/s0002-9394(14)71168-7 pmid: 7872387
Sosne G, Qiu P, Kurpakus-Wheater M, Matthew H. Thymosin beta4 and corneal wound healing: visions of the future. Ann N Y Acad Sci. 2010;1194:190-8. doi: 10.1111/j.1749-6632.2010.05472.x pmid: 20536468
Dunn SP, Heidemann DG, Chow CY, Crockford D, Turjman N, Angel J, et al. Treatment of chronic nonhealing neurotrophic corneal epithelial defects with thymosin beta4. Ann N Y Acad Sci. 2010;1194:199-206. doi: 10.1111/j.1749-6632.2010.05471.x pmid: 20536469
Morishige N, Uemura A, Morita Y, Nishida T. Promotion of Corneal Epithelial Wound Healing in Diabetic Rats by the Fibronectin-Derived Peptide PHSRN. Cornea. 2017;36(12):1544-8. doi: 10.1097/ICO.0000000000001344 pmid: 28834817
Naus CC, Giaume C. Bridging the gap to therapeutic strategies based on connexin/pannexin biology. J Transl Med. 2016;14(1):330. doi: 10.1186/s12967-016-1089-0 pmid: 27899102
Ormonde S, Chou CY, Goold L, Petsoglou C, Al-Taie R, Sherwin T, et al. Regulation of connexin43 gap junction protein triggers vascular recovery and healing in human ocular persistent epithelial defect wounds. J Membr Biol. 2012;245(7):381-8. doi: 10.1007/s00232-012-9460-4 pmid: 22797940
Schultz G, Chegini N, Grant M, Khaw P, MacKay S. Effects of growth factors on corneal wound healing. Acta Ophthalmol Suppl. 1992(202):60-6. pmid: 1322013
Wang L, Wu X, Shi T, Lu L. Epidermal growth factor (EGF)-induced corneal epithelial wound healing through nuclear factor kappaB subtype-regulated CCCTC binding factor (CTCF) activation. J Biol Chem. 2013;288(34):24363-71. doi: 10.1074/jbc.M113.458141 pmid: 23843455
Zhang Y, Akhtar RA. Epidermal growth factor stimulates phospholipase D independent of phospholipase C, protein kinase C or phosphatidylinositol-3 kinase activation in immortalized rabbit corneal epithelial cells. Curr Eye Res. 1998;17(3):294-300. doi: 10.1076/ceyr.17.3.294.5223 pmid: 9543638
Li T, Lu L. Epidermal growth factor-induced proliferation requires down-regulation of Pax6 in corneal epithelial cells. J Biol Chem. 2005;280(13):12988-95. doi: 10.1074/jbc.M412458200 pmid: 15659382
Kitazawa T, Kinoshita S, Fujita K, Araki K, Watanabe H, Ohashi Y, et al. The Mechanism of Accelerated Corneal Epithelial Healing by Human Epidermal Growth Factor. Invest Ophthalmol Vis Sci. 1990;31(9):1773-8.
Pastor JC, Calonge M. Epidermal growth factor and corneal wound healing. A multicenter study. Cornea. 1992;11(4):311-4. pmid: 1424650
Sheardown H, Wedge C, Chou L, Apel R, Rootman DS, Cheng YL. Continuous epidermal growth factor delivery in corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 1993;34(13):3593-600. pmid: 8258517
Hori K, Sotozono C, Hamuro J, Yamasaki K, Kimura Y, Ozeki M, et al. Controlled-release of epidermal growth factor from cationized gelatin hydrogel enhances corneal epithelial wound healing. J Control Release. 2007;118(2):169-76. doi: 10.1016/j.jconrel.2006.12.011 pmid: 17289206
Yu FS, Yin J, Xu K, Huang J. Growth factors and corneal epithelial wound healing. Brain Res Bull. 2010;81(2-3):229-35. doi: 10.1016/j.brainresbull.2009.08.024 pmid: 19733636
Trosan P, Svobodova E, Chudickova M, Krulova M, Zajicova A, Holan V. The key role of insulin-like growth factor I in limbal stem cell differentiation and the corneal wound-healing process. Stem Cells Dev. 2012;21(18):3341-50. doi: 10.1089/scd.2012.0180 pmid: 22873171
Nishida T, Chikama T, Morishige N, Yanai R, Yamada N, Saito J. Persistent epithelial defects due to neurotrophic keratopathy treated with a substance p-derived peptide and insulin-like growth factor 1. Jpn J Ophthalmol. 2007;51(6):442-7. doi: 10.1007/s10384-007-0480-z pmid: 18158595
Yamada N, Matsuda R, Morishige N, Yanai R, Chikama TI, Nishida T, et al. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol. 2008;92(7):896-900. doi: 10.1136/bjo.2007.130013 pmid: 18511539
Fai S, Ahem A, Mustapha M, Mohd Noh U, Bastion M. Randomized Controlled Trial of Topical Insulin for Healing Corneal Epithelial Defects Induced During Vitreoretinal Surgery in Diabetics. Asia-Pacific J Ophthalmol. 2017;6(5):418-24. doi: 10.22608/apo.201780 pmid: 28828764
Bastion M, Ling KP. Topical insulin for healing of diabetic epithelial defects?: A retrospective review of corneal debridement during vitreoretinal surgery in Malaysian patients. Med J Malaysia. 2013;68(3):209.
Zagon IS, Sassani JW, McLaughlin PJ. Insulin treatment ameliorates impaired corneal reepithelialization in diabetic rats. Diabetes. 2006;55(4):1141-7. doi: 10.2337/diabetes.55.04.06.db05-1581 pmid: 16567540
Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch Ophthalmol. 2007;125(8):1082-8. doi: 10.1001/archopht.125.8.1082 pmid: 17698755
Han X, Chen X, Liu Y, Kam W, Zhang Y, Sullivan D, et al. The Effect of Human Growth Hormone on Corneal Wound Healing in Mice. EC OPHTHALMOLOGY 2018;9:87-93.
Unterlauft JD, Kohlhaas M, Hofbauer I, Kasper K, Geerling G. [Albumin eye drops for treatment of ocular surface diseases]. Ophthalmologe. 2009;106(10):932-7. doi: 10.1007/s00347-009-2057-3 pmid: 19813016
Schargus M, Kohlhaas M, Unterlauft JD. Treatment of severe ocular surface disorders with albumin eye drops. J Ocul Pharmacol Ther. 2015;31(5):291-5. doi: 10.1089/jop.2014.0161 pmid: 25853388
Utine C, Engin Durmaz C, Koçak N. Corneal matrix repair therapy with the regenerating agent in neurotrophic persistent epithelial defects. Int J Ophthalmol. 2017;10(12):1935-9. doi: 10.18240/ijo.2017.12.25
Aifa A, Gueudry J, Portmann A, Delcampe A, Muraine M. Topical treatment with a new matrix therapy agent (RGTA) for the treatment of corneal neurotrophic ulcers. Invest Ophthalmol Vis Sci. 2012;53(13):8181-5. doi: 10.1167/iovs.12-10476 pmid: 23150626
Sevik MO, Turhan SA, Toker E. Topical Treatment of Persistent Epithelial Defects with a Matrix Regenerating Agent. J Ocul Pharmacol Ther. 2018;34(9):621-7. doi: 10.1089/jop.2018.0025 pmid: 30312119
Kymionis GD, Liakopoulos DA, Grentzelos MA, Diakonis VF, Klados NE, Tsoulnaras KI, et al. Combined topical application of a regenerative agent with a bandage contact lens for the treatment of persistent epithelial defects. Cornea. 2014;33(8):868-72. doi: 10.1097/ICO.0000000000000169 pmid: 24937169
Murri MS, Moshirfar M, Birdsong OC, Ronquillo YC, Ding Y, Hoopes PC. Amniotic membrane extract and eye drops: a review of literature and clinical application. Clin Ophthalmol. 2018;12:1105-12. doi: 10.2147/OPTH.S165553 pmid: 29950805
Shayan Asl N, Nejat F, P M, Nekoukar A, Hesam S, Ebrahimi M, et al. Amniotic Membrane Extract Eye Drop Promotes Limbal Stem Cell Proliferation and Corneal Epithelium Healing. Cell J. 2019;20(4):459-68. doi: 10.22074/cellj.2019.5423 pmid: 30123991
Baradaran-Rafii A, Asl NS, Ebrahimi M, Jabbehdari S, Bamdad S, Roshandel D, et al. The role of amniotic membrane extract eye drop (AMEED) in in vivo cultivation of limbal stem cells. Ocul Surf. 2018;16(1):146-53. doi: 10.1016/j.jtos.2017.11.001 pmid: 29104070
Elbaz U, Bains R, Zuker RM, Borschel GH, Ali A. Restoration of corneal sensation with regional nerve transfers and nerve grafts: a new approach to a difficult problem. JAMA Ophthalmol. 2014;132(11):1289-95. doi: 10.1001/jamaophthalmol.2014.2316 pmid: 25010775
Fung SSM, Catapano J, Elbaz U, Zuker RM, Borschel GH, Ali A. In Vivo Confocal Microscopy Reveals Corneal Reinnervation After Treatment of Neurotrophic Keratopathy With Corneal Neurotization. Cornea. 2018;37(1):109-12. doi: 10.1097/ICO.0000000000001315 pmid: 29053558
Ting DSJ, Figueiredo GS, Henein C, Barnes E, Ahmed O, Mudhar HS, et al. Corneal Neurotization for Neurotrophic Keratopathy: Clinical Outcomes and In Vivo Confocal Microscopic and Histopathological Findings. Cornea. 2018;37(5):641-6. doi: 10.1097/ICO.0000000000001522 pmid: 29373338
Terzis JK, Dryer MM, Bodner BI. Corneal neurotization: a novel solution to neurotrophic keratopathy. Plast Reconstr Surg. 2009;123(1):112-20. doi: 10.1097/PRS.0b013e3181904d3a pmid: 19116544
Labetoulle M, Baudouin C, Calonge M, Merayo-Lloves J, Boboridis KG, Akova YA, et al. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019;97(2):137-45. doi: 10.1111/aos.13844 pmid: 30225941
Bonini S, Lambiase A, Rama P, Sinigaglia F, Allegretti M, Chao W, et al. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology. 2018;125(9):1332-43. doi: 10.1016/j.ophtha.2018.02.022 pmid: 29653858
Sacchetti M, Bruscolini A, Lambiase A. Cenegermin for the treatment of neurotrophic keratitis. Drugs Today (Barc). 2017;53(11):585-95. doi: 10.1358/dot.2017.53.11.2722395 pmid:29451275
- Abstract Viewed: 1041 times
- Free Full Text PDF Downloaded: 849 times


