Topical Nepafenac in Treatment of Acute Central Serous Chorioretinopathy
Medical hypothesis discovery and innovation in ophthalmology,
Vol. 2 No. 4 (2013),
1 December 2013
,
Page 96-101
Abstract
This study had been performed to investigate the anatomic and functional outcomes of nepafenac 0.1% therapy in acute central serous chorioretinopathy (CSC). The medical records of 30 patients with acute CSC were reviewed for a total of 31 eye charts. Seventeen eye records of 16 patients who were treated with topical nepafenac 0.1% three times daily for four weeks and continued until complete resolution of subretinal fluid were appraised. Fourteen patients with acute CSC (a total of 14 eye records) who did not receive treatment served as the control group also had been recorded. The proportion of eyes with complete resolution of subretinal fluid, serial changes in the mean best corrected visual acuity (BCVA), and the mean central foveal thickness (CFT) at 6 months of therapy were the outcomes measured. Mean age was 42.6±8.2 years in the treatment group and 41.1±7.1 years in the control group (p=0.85). At 6 months, 14 eyes (82.3%) in the treatment group and 6 eyes (42.8%) in the control group revealed a complete resolution in the subretinal fluid (p=0.02). In the treatment group, mean BCVA (LogMAR) significantly improved from 0.19±0.17 at baseline to 0.09±0.12 at 6 months (p=0.01). In the control group, mean BCVA (LogMAR) was 0.13±0.14 at baseline and decreased to 0.1±0.11 at 6 months (p=0.28). In the treatment group, mean CFT was 349±115 µm at baseline and significantly improved to 221±95 µm at 6 months (p<0.01). In the control group, mean CFT declined from 391±138 µm at baseline to 301±125 µm at 6 months (p=0.06). No treatment-related ocular or systemic side effects were observed. In conclusion, nepafenac 0.1% has the potential to treatment acute CSC. Further trials are warranted to study its safety and efficacy for this disease.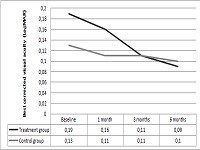
References
Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina 1994;14(3):231-42. PMID: 7973118
Wang M, Munch IC, Hasler PW, Prünte C, Larsen M. Central serous chorioretinopathy. ActaOphthalmol. 2008 Mar;86(2):126-45. PMID: 17662099
Levine R, Brucker AJ, Robinson F. Long-term follow-up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology. 1989 Jun;96(6):854-9. PMID: 2740080
Loo RH, Scott IU, Flynn HW Jr, Gass JD, Murray TG, Lewis ML, Rosenfeld PJ, Smiddy WE. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002 Feb;22(1):19-24. PMID: 11884873
Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984;68:815-20.
Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Ho A, Orlock D. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994 Aug;112(8):1057-62. PMID: 8053819
Prünte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996 Jan;121(1):26-34. PMID: 8554078
Tittl MK, Spaide RF, Wong D, Pilotto E, Yannuzzi LA, Fisher YL, Freund B, Guyer DR, Slakter JS, Sorenson JA. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 1999 Jul;128(1):63-8. PMID: 10482095
Gamache DA, Graff G, Brady MT, Spellman JM, Yanni JM. Nepafenac, a unique nonsteroidalprodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation. 2000 Aug;24(4):357-70. PMID: 10850857
Ke TL, Graff G, Spellman JM, Yanni JM. Nepafenac, a unique nonsteroidalprodrug with potential utility in the treatment of trauma-induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriers.Inflammation. 2000 Aug;24(4):371-84. PMID: 10850858
Hariprasad SM, Akduman L, Clever JA, Ober M, Recchia FM, Mieler WF. Treatment of cystoid macular edema with the new-generation NSAID nepafenac 0.1%.ClinOphthalmol. 2009;3:147-54. PMID: 19668559
Gass JD. Pathogenesis of disciform detachment of the neuroepithelium, II: Idiopathic central serous choroidopathy. Am J Ophthalmol. 1967;63:587-615.
Ficker L, Vafidis G, While A, Leaver P. Long term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1988 Nov;72(11):829-34. PMID: 3061449
Spitznas M. Central serous chorioretinopathy. In: Ryan SJ, ed. Retina. St Louis, MO: Mosby; 1989. P. 217-227.
Ober MD, Yannuzzi LA, Do DV, Spaide RF, Bressler NM, Jampol LM, Angelilli A, Eandi CM, Lyon AT. Photodynamic therapy for focal retinal pigment epithelial leaks secondary to central serous chorioretinopathy.Ophthalmology. 2005 Dec;112(12):2088-94. PMID: 16325707
Artunay O, Yuzbasioglu E, Rasier R, Sengul A, Bahcecioglu H. Intravitrealbevacizumab in treatment of idiopathic persistent central serous chorioretinopathy: a prospective, controlled clinical study. Curr Eye Res. 2010 Feb;35(2):91-8. PMID: 20136418
Lim JW, Ryu SJ, Shin MC. The effect of intravitrealbevacizumab in patients with acute central serous chorioretinopathy.Korean J Ophthalmol. 2010 Jun;24(3):155-8. PMID: 20532141
Pikkel J, Beiran I, Ophir A, Miller B. Acetazolamide for central serous retinopathy. Ophthalmology. 2002 Sep;109(9):1723-5. PMID: 12208723
Nielsen JS, Jampol LM. Oral mifepristone for chronic central serous chorioretinopathy.Retina. 2011 Oct;31(9):1928-36. PMID: 21878843
Meyerle CB, Freund KB, Bhatnagar P, Shah V, Yannuzzi LA. Ketoconazole in the treatment of chronic idiopathic central serous chorioretinopathy.Retina. 2007 Sep;27(7):943-6. PMID: 17891021
Avci R, Deutman AF. Treatment of central serous choroidopathy with the beta receptor blocker metoprolol (preliminary results).KlinMonblAugenheilkd. 1993 Mar;202(3):199-205. PMID: 8510413
Tatham A, Macfarlane A. The use of propranolol to treat central serous chorioretinopathy: an evaluation by serial OCT. J OculPharmacolTher. 2006 Apr;22(2):145-9. PMID: 16722801
Lim JW, Kim MU, Shin MC. Aqueous humor and plasma levels of vascular endothelial growth factor and interleukin-8 in patients with central serous chorioretinopathy. Retina. 2010 Oct;30(9):1465-71. PMID: 20526231
Ratanasukon M, Bhurayanontachai P, Jirarattanasopa P. High-dose antioxidants for central serous chorioretinopathy; the randomized placebo-controlled study. BMC Ophthalmol. 2012 Jul 16;12:20. PMID: 22800086
Chan WM, Lai TY, Lai RY, Liu DT, Lam DS.Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy.Ophthalmology. 2008 Oct;115(10):1756-65. PMID: 18538401
- Abstract Viewed: 3405 times
- FULL TEXT PDF Downloaded: 2998 times


