Prevention of Corneal Neovascularization; a Preliminary Experimental Study in Rabbits
Medical hypothesis discovery and innovation in ophthalmology,
Vol. 9 No. 1 (2020),
19 November 2019
,
Page 47-55
Abstract
The purpose of this study was to compare the effects of propranolol, timolol and bevacizumab with betamethasone to prevent corneal neovascularization (CNV) in rabbits. This study was performed on 28 male rabbits. CNV was induced by three 7-0 silk sutures 2 mm long and 1 mm distal to the limbus. Animals were randomly divided into 4 groups of propranolol + betamethasone, timolol + betamethasone and bevacizumab + betamethasone and betamethasone alone. Eye drops were started from the first day of study. On 7th, 14th, 21st, 28th, 35th and 42nd days, vascular progression, time of neovascularization and vascular area were evaluated and compared with the control group (betamethasone alone). There was a significant reduction in the area of neovascularization in the timolol and bevacizumab groups compared to the control group (P-value = 0.05, P=0.047, respectively). Also, regarding vascular progression, there was a significant decrease in the timolol and bevacizumab groups (P-value = 0.014, P=0.002, respectively). Regarding delayed onset of neovascularization, there was a significant difference in the timolol and bevacizumab group in rabbits (P-value = 0.04, P=0.00, respectively). In conclusion, the use of timolol and bevacizumab drops besides betamethasone can delay neovascularization and decrease the length of corneal vascularization in rabbits.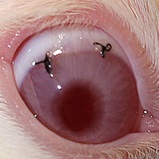
References
Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmology. 2018;66(2):190.
Feizi S, Azari AA, Safapour S. Therapeutic approaches for corneal neovascularization. Eye Vis (Lond). 2017;4:28. doi: 10.1186/s40662-017-0094-6 pmid: 29234686
Lee DS, Kim MK, Wee WR. Biometric risk factors for corneal neovascularization associated with hydrogel soft contact lens wear in Korean myopic patients. Korean J Ophthalmol. 2014;28(4):292-7. doi: 10. 3341/kjo.2014.28.4.292 pmid: 25120337
Gimenez F, Suryawanshi A, Rouse BT. Pathogenesis of herpes stromal keratitis--a focus on corneal neovascularization. Prog Retin Eye Res. 2013;33:1-9. doi: 10.1016/j.preteyeres.2012.07.002 pmid: 22892644
Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2006;104:264-302. pmid: 17471348
Chen P, Yin H, Wang Y, Wang Y, Xie L. Inhibition of VEGF expression and corneal neovascularization by shRNA targeting HIF-1alpha in a mouse model of closed eye contact lens wear. Mol Vis. 2012;18:864-73. pmid: 22511848
Gupta D, Illingworth C. Treatments for corneal neovascularization: a review. Cornea. 2011;30(8):927-38. doi: 10.1097/ICO.0b013e318201405a pmid: 21389854
Dastjerdi MH, Al-Arfaj KM, Nallasamy N, Hamrah P, Jurkunas UV, Pineda R, 2nd, et al. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol. 2009;127(4):381-9. doi: 10.1001/archophthalmol.2009.18 pmid: 19365012
Chang JH, Garg NK, Lunde E, Han KY, Jain S, Azar DT. Corneal neovascularization: an anti-VEGF therapy review. Surv Ophthalmol. 2012;57(5):415-29. doi: 10.1016/j.survophthal.2012.01.007 pmid: 22898649
Erdurmus M, Totan Y. Subconjunctival bevacizumab for corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2007;245(10):1577-9. doi: 10.1007/ s00417-007-0587-4 pmid: 17458556
Chen WL, Chen YM, Chu HS, Lin CT, Chow LP, Chen CT, et al. Mechanisms controlling the effects of bevacizumab (avastin) on the inhibition of early but not late formed corneal neovascularization. PLoS One. 2014; 9(4):e94205. doi: 10.1371/journal.pone. 0094205 pmid: 24714670
Simavli H, Erdurmus M, Terzi EH, Bucak YY, Onder HI, Kukner AS. The effect of beta receptor blockade through propranolol on corneal neovascularization. J Ocul Pharmacol Ther. 2014;30(8):650-6. doi: 10.1089/ jop.2013.0238 pmid: 24983781
Rusovici R, Sakhalkar M, Chalam KV. Evaluation of cytotoxicity of bevacizumab on VEGF-enriched corneal endothelial cells. Mol Vis. 2011;17:3339-46. pmid: 22219629
Cho YK, Shin EY, Uehara H, Ambati B. The Effect of 0.5% Timolol Maleate on Corneal(Lymph)Angiogenesis in a Murine Suture Model. J Ocul Pharmacol Ther. 2018;34(5):403-9. doi: 10.1089/jop.2017.0119 pmid: 29757062
Kasiri A, Ghomi MR, Feghhi M, Farrahi F, Mirdehghan MS, Hedayati H. Topical Timolol Inhibits Corneal Neovascularization in Rabbits. Med Hypothesis Discov Innov Ophthalmol. 2017;6(2):39-43. pmid: 29367933
Comstock TL, Decory HH. Advances in corticosteroid therapy for ocular inflammation: loteprednol etabonate. Int J Inflam. 2012;2012:789623. doi: 10.1155/2012/789623 pmid: 22536546
Oner V, Kucukerdonmez C, Akova YA, Colak A, Karalezli A. Topical and subconjunctival bevacizumab for corneal neovascularization in an experimental rat model. Ophthalmic Res. 2012;48(3):118-23. doi: 10. 1159/000337139 pmid: 22538642
Bock F, Konig Y, Kruse F, Baier M, Cursiefen C. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008;246(2):281-4. doi: 10.1007/s00417-007-0684-4 pmid: 17934753
Bhatti N, Qidwai U, Hussain M, Kazi A. Efficacy of topical bevacizumab in high-risk corneal transplant survival. Pak J Med Sci. 2013;29(2):519-22. pmid: 24353568
Rocher N, Behar-Cohen F, Pournaras JA, Naud MC, Jeanny JC, Jonet L, et al. Effects of rat anti-VEGF antibody in a rat model of corneal graft rejection by topical and subconjunctival routes. Mol Vis. 2011;17:104-12. pmid: 21245949
Gan L, Fagerholm P, Palmblad J. Vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in the regulation of corneal neovascularization and wound healing. Acta Ophthalmol Scand. 2004;82(5):557-63. doi: 10.1111/j.1600-0420.2004.00312.x pmid: 15453853
Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000;41(9):2514-22. pmid: 10937562
Luis de Redin I, Boiero C, Recalde S, Agueros M, Allemandi D, Llabot JM, et al. In vivo effect of bevacizumab-loaded albumin nanoparticles in the treatment of corneal neovascularization. Exp Eye Res. 2019;185:107697. doi: 10.1016/j.exer.2019.107697 pmid: 31228461
Baradaran-Rafii A, Ashnagar A, Heidari Keshel S, Jabbehdari S, Baradaran-Rafii G. Regression of corneal neovascularization: Adiponectin versus bevacizumab eye drops. Eur J Ophthalmol. 2019:1120672119874947. doi: 10.1177/1120672119 874947 pmid: 31523981
Sahan B, Ciftci F, Eyuboglu S, Yaba A, Yilmaz B, Yalvac BI. Comparison of the Effects of Dovitinib and Bevacizumab on Reducing Neovascularization in an Experimental Rat Corneal Neovascularization Model. Cornea. 2019;38(9):1161-8. doi: 10.1097/ICO.00000 00000002012 pmid: 31180924
Xu X, Yu J, Shi H, Zhang J, Li X. Prevention of corneal neovascularization by subconjunctival injection of avastin(R) loaded thermosensitive hydrogels in rabbit model. Int J Pharm. 2018;552(1-2):164-70. doi: 10. 1016/j.ijpharm.2018.09.017 pmid: 30217769
Lopes GJA, Casella AMB, Oguido AP, Matsuo T. Effects of topical and subconjunctival use of bevacizumab on corneal neovascularization in rabbits' eyes. Arq Bras Oftalmol. 2017;80(4):252-6. doi: 10.5935/0004-2749. 20170061 pmid: 28954027
Huang J, Wang W, Yu J, Yu X, Zheng Q, Peng F, et al. Combination of dexamethasone and Avastin((R)) by supramolecular hydrogel attenuates the inflammatory corneal neovascularization in rat alkali burn model. Colloids Surf B Biointerfaces. 2017;159:241-50. doi: 10.1016/j.colsurfb.2017.07.057 pmid: 28800463
Sella R, Gal-Or O, Livny E, Dachbash M, Nisgav Y, Weinberger D, et al. Efficacy of topical aflibercept versus topical bevacizumab for the prevention of corneal neovascularization in a rat model. Exp Eye Res. 2016;146:224-32. doi: 10.1016/j.exer.2016.03.021 pmid: 27020759
Ozdemir O, Altintas O, Altintas L, Ozkan B, Akdag C, Yuksel N. Comparison of the effects of subconjunctival and topical anti-VEGF therapy (bevacizumab) on experimental corneal neovascularization. Arq Bras Oftalmol. 2014;77(4):209-13. doi: 10.5935/0004-2749. 20140054 pmid: 25410169
Papathanassiou M, Theodoropoulou S, Analitis A, Tzonou A, Theodossiadis PG. Vascular endothelial growth factor inhibitors for treatment of corneal neovascularization: a meta-analysis. Cornea. 2013; 32(4):435-44. doi: 10.1097/ICO.0b013e3182542613 pmid: 22668582
Fraunfelder FT, Fraunfelder FW, Chambers WA. Drug-Induced Ocular Side Effects: Clinical Ocular Toxicology E-Book: Clinical Ocular Toxicology: Elsevier Health Sciences; 2014.
Gal-Or O, Livny E, Sella R, Nisgav Y, Weinberger D, Livnat T, et al. Efficacy of Subconjunctival Aflibercept Versus Bevacizumab for Prevention of Corneal Neovascularization in a Rat Model. Cornea. 2016; 35(7):991-6. doi: 10.1097/ICO.0000000000000849 pmid: 27124775
Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J Virol. 2001;75(20):9828-35. doi: 10.1128/JVI.75.20.9828-9835.2001 pmid: 11559816
Filippi L, de Libero C, Zamma Gallarati B, Fortunato P, Piozzi E. Propranolol eye drops in patients with corneal neovascularization. Medicine (Baltimore). 2018; 97(45):e13002. doi: 10.1097/MD.0000000000013002 pmid: 30407290
- Abstract Viewed: 1053 times
- Free Full Text PDF Downloaded: 542 times


