Non-inferiority evaluation of preservative-free latanoprost/timolol eye drops solution versus preserved latanoprost/timolol eye drops in patients with high intraocular pressure and open-angle glaucoma
Medical hypothesis discovery and innovation in ophthalmology,
Vol. 9 No. 4 (2020),
28 February 2021
,
Page 255-263
https://doi.org/10.51329/mehdiophthal1411
Abstract
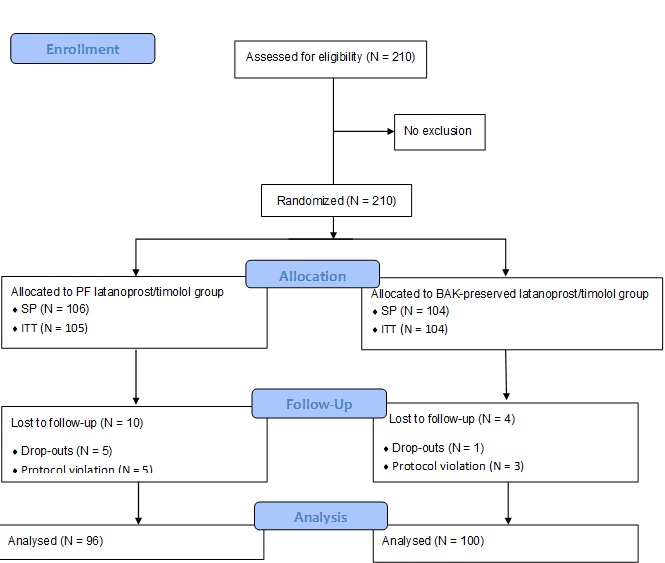
Background: This study aimed to evaluate the non-inferiority and safety of a newly developed preservative-free (PF) multi-dose latanoprost/timolol ophthalmic solution, compared with the benzalkonium chloride (BAK)-preserved fixed combination, in patients with open-angle glaucoma and ocular hypertension.Methods: A Phase III randomized multi-center observer-blind parallel-group clinical trial was conducted. A total of 210 adult patients (aged over 18 years) were randomly treated with the PF- or the BAK-preserved latanoprost/timolol solution once daily in the affected eye(s) for 12 weeks. Follow-up visits were scheduled at weeks 2, 6, and 12; intraocular pressure (IOP) was recorded at 8:00 AM, 12:00 PM, and 4:00 PM. The primary efficacy endpoint to prove non-inferiority was the IOP change at 8:00 AM (± 1 hour) from the baseline to the end of treatment (week 12) in the studied eye. Safety parameters were also assessed.
Results: In total, 196 patients completed the study. The pressure-lowering effect of the PF eye drops was comparable to that of the preserved formulation at all time points. Latanoprost/timolol PF formulation was non-inferior to the BAK-preserved solution as shown by the change in IOP from day 0 to week 12. The point estimate of the inter-treatment difference was 0.624 mmHg (95% CI: -0.094, 1.341). Both treatments were well-tolerated during the study, and they had similar adverse event profiles.
Conclusions: PF-latanoprost/timolol combination was found to be non-inferior to the BAK-preserved formulation based on the efficacy at all times, with similar local tolerance.
- Abstract Viewed: 285 times
- Full Text PDF Downloaded: 204 times


